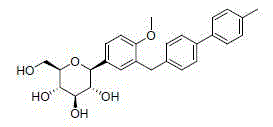
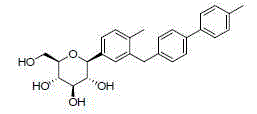
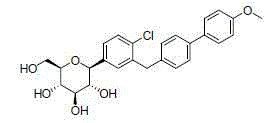
C-triaryl glucoside SGLT-2 inhibitor
A technology of glucoside and alkyl, applied in the field of medicine, can solve the problems of low bioavailability and anti-diabetic drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Example 1 Preparation of Compound 1
[0082]
[0083] Preparation of Compound 1-3:
[0084] Add 70.6 g of 2-chloro-5-bromobenzoic acid (1-1) and 500 mL of tetrahydrofuran into a round-bottomed flask, and slowly add 200 mL of 2M borane dimethyl sulfide complex dropwise at 0°C; 25°C) and stirred overnight; at 0°C, methanol was slowly added dropwise until no bubbles emerged; the reaction liquid was concentrated and evaporated to dryness under reduced pressure; 300 mL of water and 300 mL of ethyl acetate were added to the residue, and the layers were extracted, and the organic phase was washed with saturated brine Once, dried with anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness to obtain compound 1-2, which was directly carried out to the next step without purification.
[0085] Add 60g PCC and 60g silica gel powder into a round bottom flask, mix well, add 500mL dichloromethane, cool to 0°C, add dropwise 45g of compound 1-2 in dichlorome...
Embodiment 2
[0098] Example 2 Preparation of Compound 2
[0099]
[0100] Preparation of intermediate 4-bromo-4'-fluorobiphenyl
[0101] Add 4 g of p-bromoiodobenzene, 2.9 g of 4-fluorophenylboronic acid, 0.84 g of tetrakis(triphenylphosphine) palladium, 5.8 g of potassium carbonate and 100 mL of toluene or N,N-dimethylmethane into a 250 mL round bottom flask amides. Heated to 100°C under the protection of nitrogen, and detected the progress of the reaction with a thin-layer chromatography plate. After the reaction was completed, add 100 mL of water and 150 mL of ethyl acetate to separate the liquids. The organic phase was washed with 1N hydrochloric acid and saturated brine respectively, dried over anhydrous sodium sulfate, evaporated to dryness, and purified by silica gel column chromatography (petroleum ether as the eluent) to obtain 4- Bromo-4'-fluorobiphenyl 1.6 g, 46% yield, MS m / z (ESI) 272.9 [M+Na] + .
[0102] The preparation method of compound 2 refers to Example 1, and th...
Embodiment 3
[0105] Example 3 Preparation of compound 3
[0106]
[0107] The preparation method of intermediate 4-bromo-4'-chlorobiphenyl refers to the preparation of 4-bromo-4'-fluorobiphenyl in Example 2, except that 4-chlorophenylboronic acid is used instead of 4-fluorophenylboronic acid.
[0108] The preparation method of compound 3 refers to Example 1, and the intermediate 4-bromo-4'-chlorobiphenyl is used to replace the 4-bromobiphenyl in Example 1, and the total yield is 30%.
[0109] 1 H NMR (400 MHz, DMSO-d 6 ) δ7.58-7.56(m, 2H), 7.49-7.48(m, 2H), 7.47-7.46(m, 2H), 7.40-7.37(m, 2H), 7.28-7.23(m, 3H), 4.94( m, 2H), 4.84(d, J=6.0 Hz, 2H), 4.43(m, 2H), 4.14-3.99(m, 3H), 3.70-3.66(m, 1H), 3.46-3.40(m,1H) , 3.27-3.08(m, 4H).
[0110] MS m / z (ESI) 498.1 [M+Na] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com