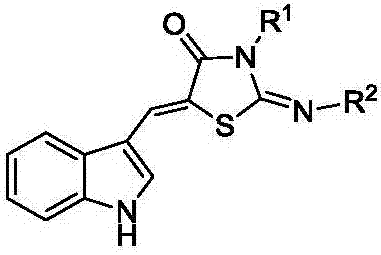
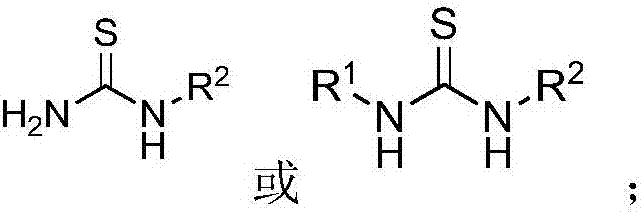
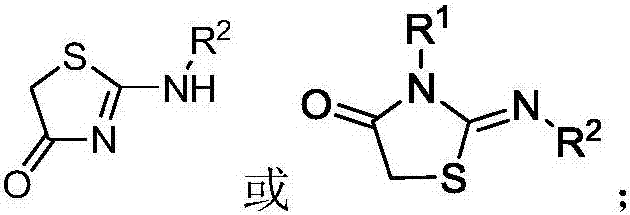
A kind of 5-(1h-indole-3-methylene)-1,3-thiazolidin-4-one derivative and its synthesis method and application
A technology for imino thiazolidine and derivatives, which is applied in the field of 5--1,3-thiazolidine-4-type derivatives and their synthesis, can solve the problems of low cell membrane permeability and the like, achieves good inhibitory effect, The effect of good IC50 value and good inhibition rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The first synthetic method is a general synthetic method of two-step reaction, that is, firstly forming a thiazole heterocycle and then introducing an extracyclic 5-double bond, which specifically includes the following steps:
[0043] 1) Synthesis of iminothiazolidin-4-one derivatives, iminothiazolidin-4-one derivatives are synthesized by one of the following two methods:
[0044] Method 1: Mix the thiourea compound and ethyl chloroacetate and dissolve it in ethanol, then add anhydrous sodium acetate or not, reflux for 1 to 10 hours, and the reaction solution obtained after the reaction is post-treated , to obtain iminothiazolidin-4-one derivatives (see formula 7 for unsubstituted and monosubstituted synthetic routes, see formula 8 for disubstituted ones); wherein, thiourea compounds, ethyl chloroacetate and anhydrous The molar ratio of sodium acetate is 1:(1~1.5):(1~1.5), and the thiourea compound is thiourea, N,N'-disubstituted thiourea or N-monosubstituted thiourea;...
Embodiment 1
[0059] 1) Synthesis of 2-imino-1,3-thiazolidin-4-one:
[0060] In a clean 50mL single-necked bottle, add 0.05mol thiourea and 25.0mL ethanol with a mass concentration of 95%, reflux and stir for about 20min to completely dissolve the thiourea, add 0.05mol ethyl chloroacetate dropwise within 10min, and then stir Reflux reaction for 3 hours, a large number of white fine crystals appeared in the obtained reaction solution, the reaction solution was cooled to room temperature, then suction filtered, washed with a small amount of ethanol, and finally dried to obtain a white solid 2-imino-1,3-thiazolidine- 4-Kone (7.40g), yield 92.1%, m.p.222-224°C.
[0061] 2) Synthesis of 2-imino-5-(1H-indol-3-yl)methylene-1,3-thiazolidin-4-one (A1):
[0062] Weigh 1.00mmol of 2-imino-1,3-thiazolidin-4-one and 1.00mmol of 1H-indole-3-carbaldehyde into a clean 50mL flask, add 8.0mL of absolute ethanol, stir, and add 0.2mL Anhydrous piperidine, heated to reflux for 4.0 hours, stopped heating, and ...
Embodiment 2
[0064] 1) Synthesis of 2-phenylmethylene-1,3-thiazolidin-4-one:
[0065] In a clean 50mL single-necked bottle, add 2.0mmol N-phenylthiourea, 8.0mL ethanol with a mass concentration of 95%, add 2.63mmol anhydrous sodium acetate and 2.60mmol ethyl chloroacetate under stirring, and gradually raise the temperature and reflux the reaction After 6.0 hours, the heating was stopped, and a large number of fine white crystals appeared in the obtained reaction solution. The reaction solution was cooled to room temperature to further precipitate solids, and suction filtered. The obtained filter cake was washed with a small amount of ethanol, and dried to obtain light yellow solid 2-phenylmethylene- 1,3-Thiazolidin-4-one (0.36g), m.p.176-178°C, yield 82.0%.
[0066] 2) Synthesis of 2-phenylimino-5-(1H-indol-3-yl)methylene-1,3-thiazolidin-4-one (A2)
[0067] Weigh 1.90mmol 2-phenylimino-1,3-thiazolidin-4-one, 1.90mmol 1H-indole-3-carbaldehyde in a clean 50mL flask, add 14.0mL absolute etha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com