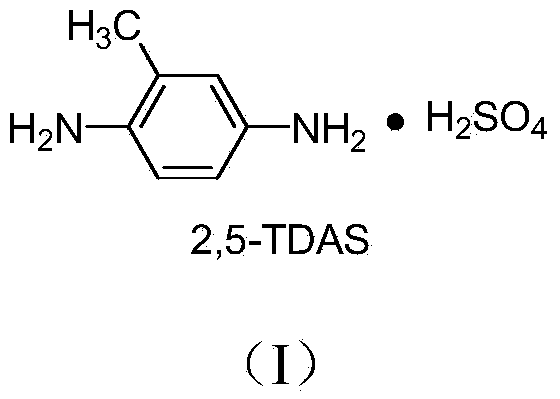
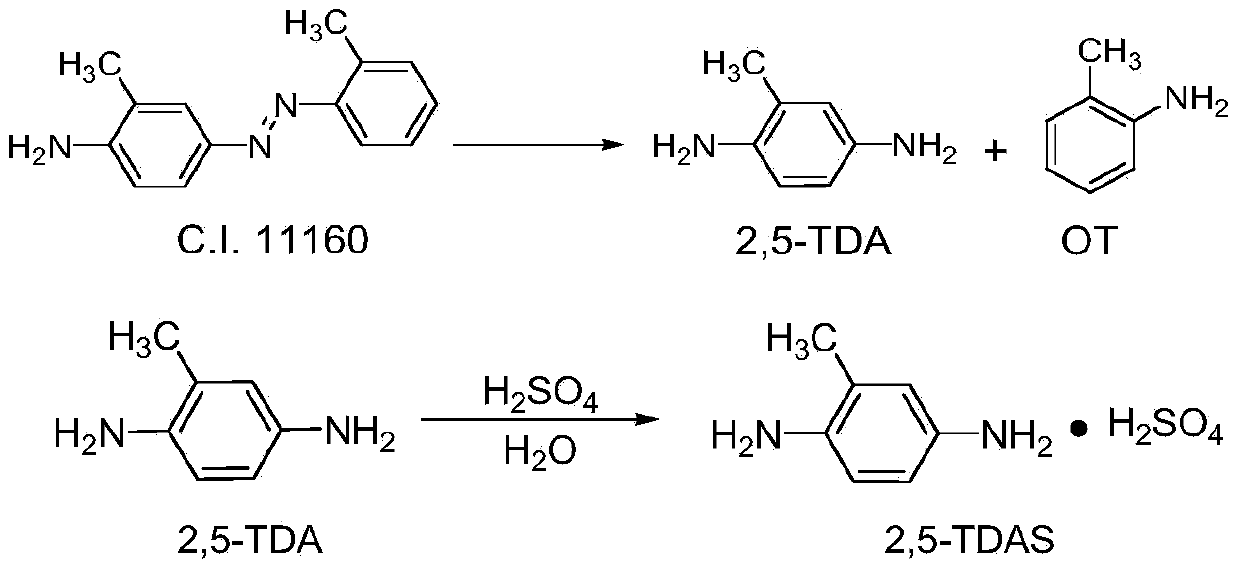
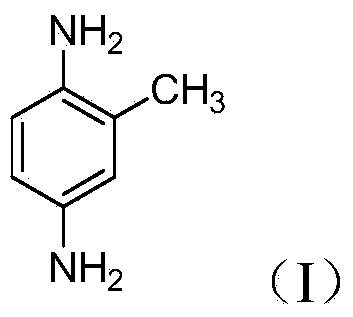
Preparation method of 2,5-diaminotoluene sulfate
A technology of diaminotoluene and aminoazotoluene, which is applied in the field of preparation of 2,5-diaminotoluene sulfate, can solve the problems of high loss rate and no OT removal, and achieve less loss, less side reactions, and easy The effect of industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 A preparation method of 2,5-diaminotoluene sulfate (direct extraction and adsorption process for removing OT), comprising the following steps in sequence:
[0048] 1) Put 90g of reduced iron powder and 250ml of water into a 500ml three-necked flask equipped with a thermometer, a reflux tube and a stirring device. After the temperature rises to 90°C, slowly add about 17g of concentrated hydrochloric acid dropwise, and keep the temperature after the dropwise addition. After the catalyst was activated for about 30 minutes, 76.9 g of 2-aminoazotoluene (C.I.11160) (0.341 mol) was added to the reactor, and the addition was completed in about 10 minutes. After the reaction distillation device was installed on the reaction device, the temperature was raised to reflux for reaction. The temperature is 95-102°C. During the reaction process, oily matter is gradually produced, which evaporates with water vapor, condenses, and then flows into the collection bottle. After th...
Embodiment 2-5
[0052] Table 1 Effect of the type and amount of metal and acid on the product
[0053]
Embodiment 6-10
[0055] Table 2 The effect of the type of extractant and the number of extractions on the product
[0056]
[0057] In summary, as shown in Tables 1 and 2, in Examples 1-10 of the present invention, except for Examples 8 and 9, the purity of the products obtained is greater than 99.5%, the yield is more than 70%, and the OT content is not greater than 30ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com