Method for preparing high-purity (3,4-diaminophenyl)(4-fluorophenyl) ketone by virtue of catalytic hydrogenation
A diaminophenyl, catalytic hydrogenation technology, applied in the direction of chemical instruments and methods, preparation of organic compounds, physical/chemical process catalysts, etc., can solve the problem of difficult treatment of sulfide-containing wastewater, low product yield, and easy production of active nickel Fire and other problems, to achieve the effect of inhibiting carbonyl from being reduced to methylene and defluorination, simple preparation method, and high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
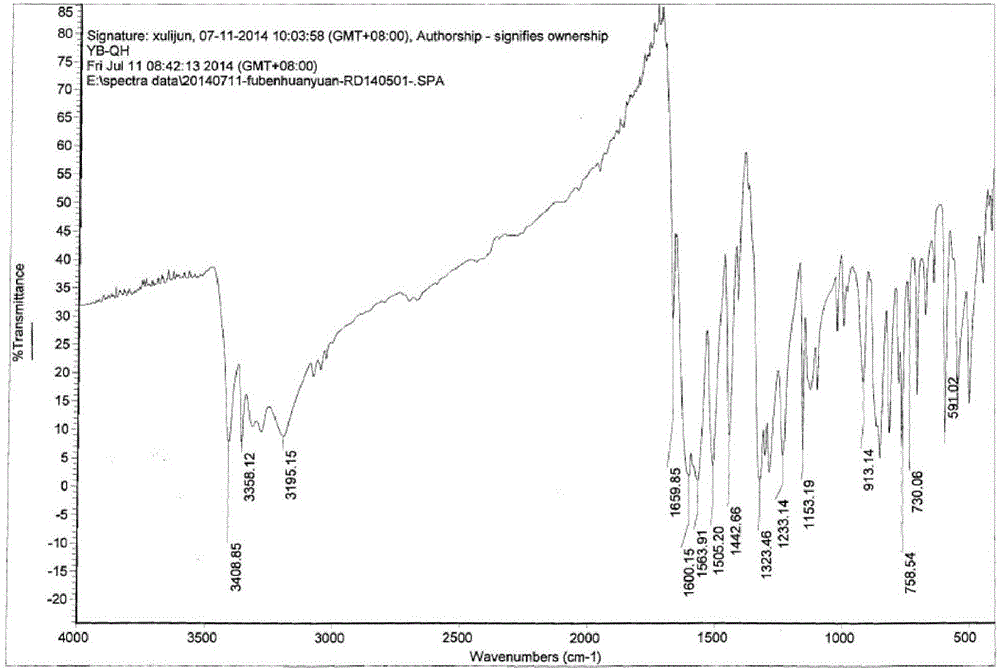
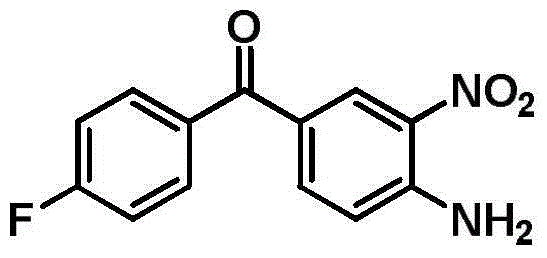
[0020] Catalytic hydrogenation: In a 1L autoclave, add 75.0g (4-amino-3-nitrophenyl) (4-fluorophenyl) ketone, 300ml methanol, 0.5g3% Pt / C catalyst (Jiangxi Hanshi Platinum Industry Co., Ltd.), then cover the lid of the kettle, replace with nitrogen for 3 times, then replace with hydrogen for 3 times, stir and heat up to 50°C, start to introduce hydrogen, so that the temperature in the kettle does not exceed 60°C, and the pressure is maintained at 0.4-0.6MPa , when the hydrogen is no longer absorbed, keep the pressure in the kettle at 0.6MPa, continue the reaction for 1h, cool down, release the pressure, quickly extract the reaction liquid in the kettle, filter the catalyst, recover 75% of methanol, pour the remaining liquid into 750ml In the water, a bright yellow solid was precipitated, filtered, and vacuum-dried (60°C) to obtain 62.8 g of (3,4-diaminophenyl)(4-fluorophenyl)ketone, with a yield of 94.6%. Product liquid chromatography-mass spectrometer analysis, the content of...
Embodiment 2
[0022] Catalyst preparation: 0.135g H 2 PtCl 6 and 0.03g RuCl 3 Dissolve in 30ml of water, soak 1g of activated carbon in this solution, soak for 10 hours, heat to 95°C, add NaOH solution and glacial acetic acid dropwise, heat and reduce for 1 hour, after the reduction is completed, cool, filter, and wash with water until neutral to obtain Pt-Ru / C catalyst.
[0023] Catalytic hydrogenation: In a 1L autoclave, add 75.0g (4-amino-3-nitrophenyl) (4-fluorophenyl) ketone, 300ml methanol, 0.5g Pt-Ru / C catalyst, and then cover the kettle Cover, replace with nitrogen for 3 times, then replace with hydrogen for 3 times, stir and raise the temperature to 50°C, start to introduce hydrogen, so that the temperature in the kettle does not exceed 60°C, keep the pressure at 0.4-0.6MPa, when the hydrogen is no longer absorbed, keep The pressure in the kettle is 0.6MPa, continue to react for 1h, cool down, release the pressure, quickly extract the reaction liquid in the kettle, filter the ca...
Embodiment 3
[0025] Catalyst preparation: 0.135g H 2 PtCl 6 and 0.4g TiCl 4 Dissolve in 30ml of water, soak 1g of activated carbon in this solution for 12 hours, heat to 90°C, add KBH dropwise 4 The solution was heated and reduced for 1.5 hours. After the reduction was completed, it was cooled, filtered, and washed with water until neutral to obtain a Pt-Ti / C catalyst.
[0026] Catalytic hydrogenation: In a 1L autoclave, add 80.0g (4-amino-3-nitrophenyl) (4-fluorophenyl) ketone, 480ml toluene, 1.2g Pt-Ti / C catalyst, and then cover the kettle Cover, replace with nitrogen for 3 times, then replace with hydrogen for 3 times, stir and raise the temperature to 50°C, start to introduce hydrogen, so that the temperature in the kettle does not exceed 60°C, keep the pressure at 0.5-0.9MPa, when the hydrogen is no longer absorbed, keep The pressure in the kettle is 1.0MPa, continue to react for 1h, cool down, release the pressure, quickly extract the reaction liquid in the kettle, filter the cata...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com