Zinc coordination polymer as well as preparation method and application thereof
A zinc coordination polymer and reaction technology, applied to zinc coordination polymer, its preparation and application fields, can solve problems such as low recycling rate, and achieve the effects of reducing degradation time, stable structure and improving catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment one: the synthesis of zinc coordination polymer
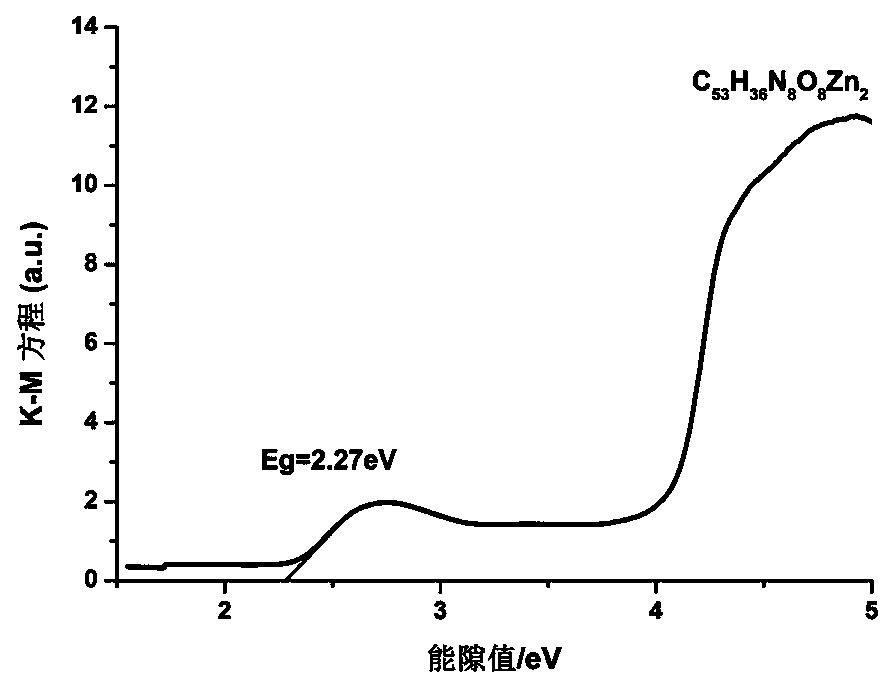
[0043] Take zinc nitrate hexahydrate Zn(NO 3 ) 2 ·6H 2 O (0.030g, 0.1mmol), 1,3-H isophthalic acid 2 BDC (0.016g, 0.1mmol) and tetrakis[4-(1-imidazolyl)phenyl]methane (0.029g, 0.05mmol) were placed in an 8mL heat-resistant glass tube, and 3mL of a mixed solvent with a volume ratio of 1:1 was added After blocking with acetonitrile / water. React in an oven at a constant temperature of 150°C for 2 days, slowly lower to room temperature at a rate of 5°C / hour, filter, wash with distilled water, and dry to obtain a yellow crystal as product 1, which is a zinc coordination polymer with the molecular formula C 53 h 36 N 8 o 8 Zn 2 .
[0044] Product 1 is carried out infrared analysis, and the result is as follows:
[0045] IR:v(KBr) / cm -1 3447m, 3136w, 1613s, 1561w, 1523s, 1373s, 1313w, 1270w, 1126w, 1068m, 966m, 827m, 762s, 656m, 561m.
[0046] The structure of the product 1 was analyzed by X-ray single cr...
Embodiment 2
[0050] Embodiment two: the synthesis of the zinc coordination polymer of Co ion doping
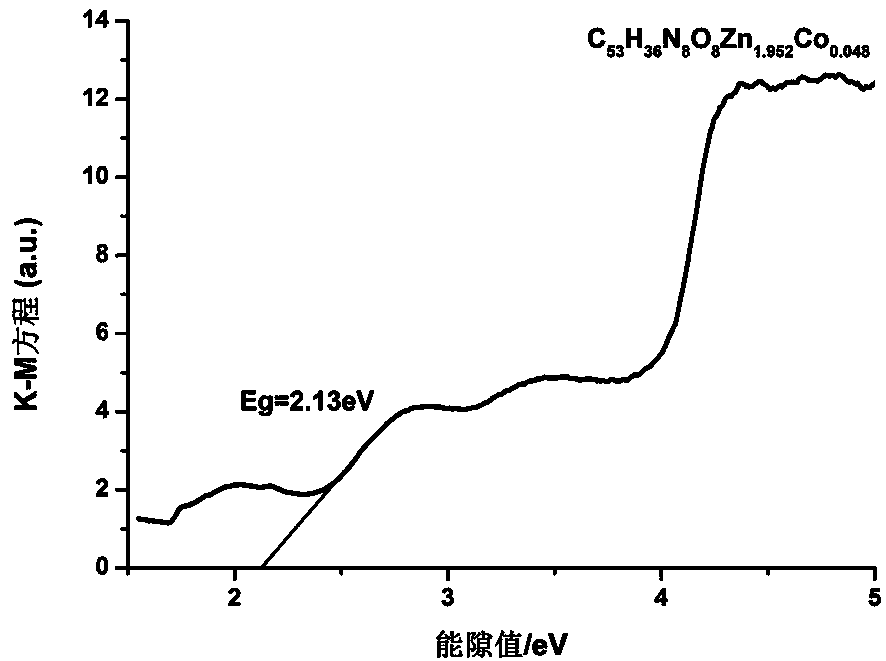
[0051] Zinc coordination polymer C 53 h 36 N 8 o 8 Zn 2 placed in cobalt nitrate Co(NO 3 ) 2 ·6H 2 O in acetonitrile / water mixed solution, sealed in a heat-resistant glass tube, kept in an oven at 80°C for 24 hours, filtered, washed with distilled water, and dried to obtain an orange compound, which is a zinc coordination polymer doped with cobalt ions , the molecular formula is C 53 h 36 N 8 o 8 Zn 1.952 co 0.048 . ICP analysis found that the cobalt ion-doped zinc coordination polymer contained 97.60% Zn 2+ and 2.40% Co 2+ . Zinc coordination polymer C doped with cobalt ions 53 h 36 N 8 o 8 Zn 1.952 co 0.048 Carry out infrared analysis, the result is as follows:
[0052] IR:v(KBr) / cm -1 3445m, 3133w, 1615s, 1562w, 1523s, 1373s, 1314w, 1270w, 1126w, 1068m, 966m, 826m, 762s, 656m, 561m.
[0053] Using X-ray single crystal diffraction to study the cobalt ion-doped zi...
Embodiment 3
[0057] Example 3: Photodegradation of Rhodamine B Catalyzed by Zinc Coordination Polymer
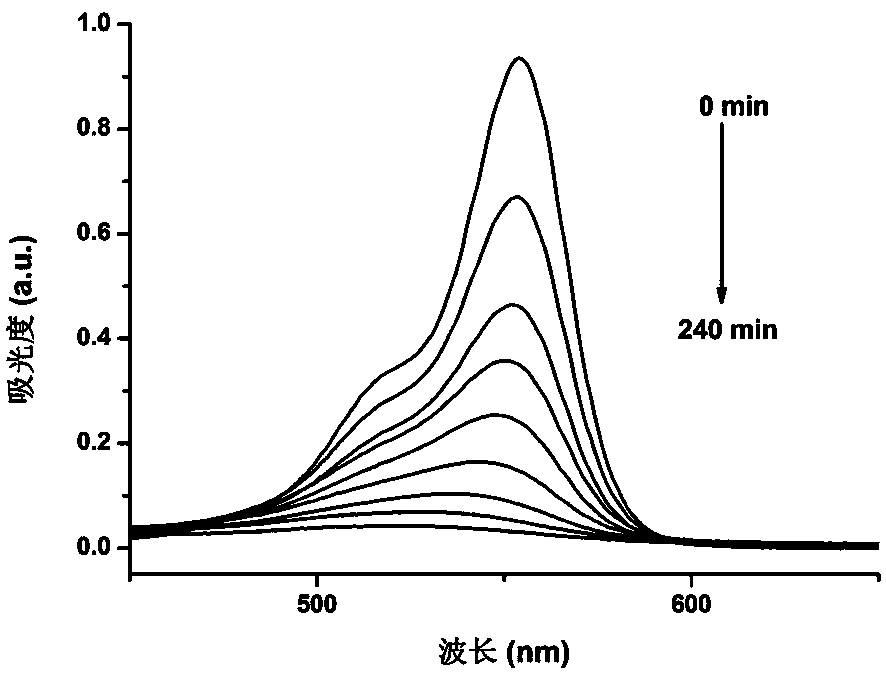
[0058] Take 10 mg of zinc coordination polymer C 53 h 36 N 8 o 8 Zn 2 Concentration in 50mL is 3×10 -4 mol L -1 In the rhodamine B aqueous solution, the catalytic photodegradation reaction was carried out under a 400W ultraviolet lamp, and 1mL was sampled every 30min, diluted to 10mL with distilled water to measure the ultraviolet absorption. attached image 3 It is the ultraviolet absorption spectrogram of the rhodamine B aqueous solution. It can be seen that it takes 240 minutes for the zinc coordination polymer to catalyze the photodegradation of rhodamine B.
[0059] After the degradation was completed, the zinc coordination polymer was recovered by filtration, washing, and drying, and the experiment of catalytic photodegradation of rhodamine B was carried out again. attached Figure 4 It is the change figure of the concentration of cycle number and rhodamine B, and the ordi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com