Fusion protein containing leucine-rich repetitive sequence, and preparation method and application thereof
A fusion protein and protein technology, applied in the field of fusion proteins, can solve the problems of difficulty in protein purification, cumbersome processes and steps, inaccurate expression of target proteins, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
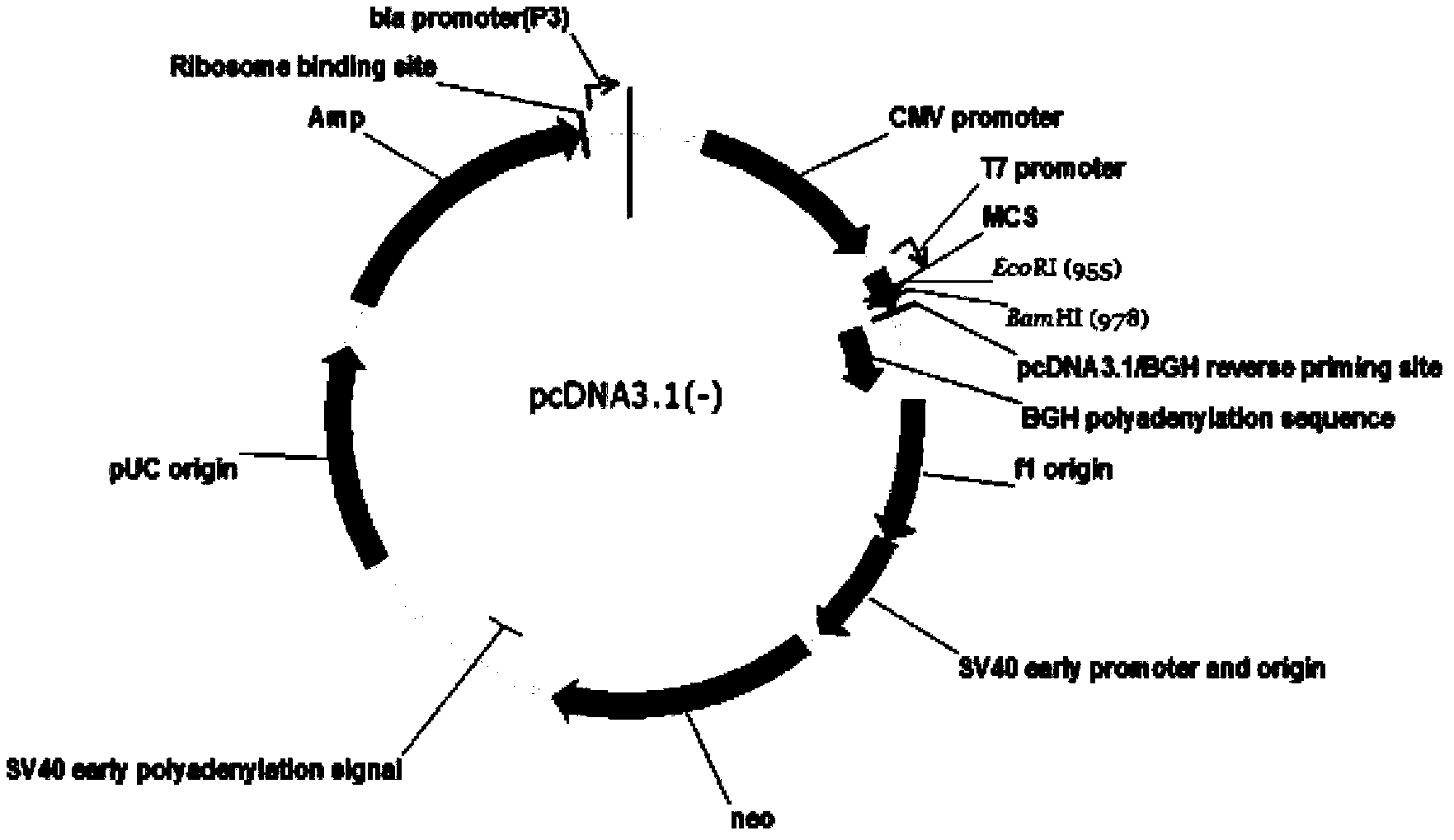
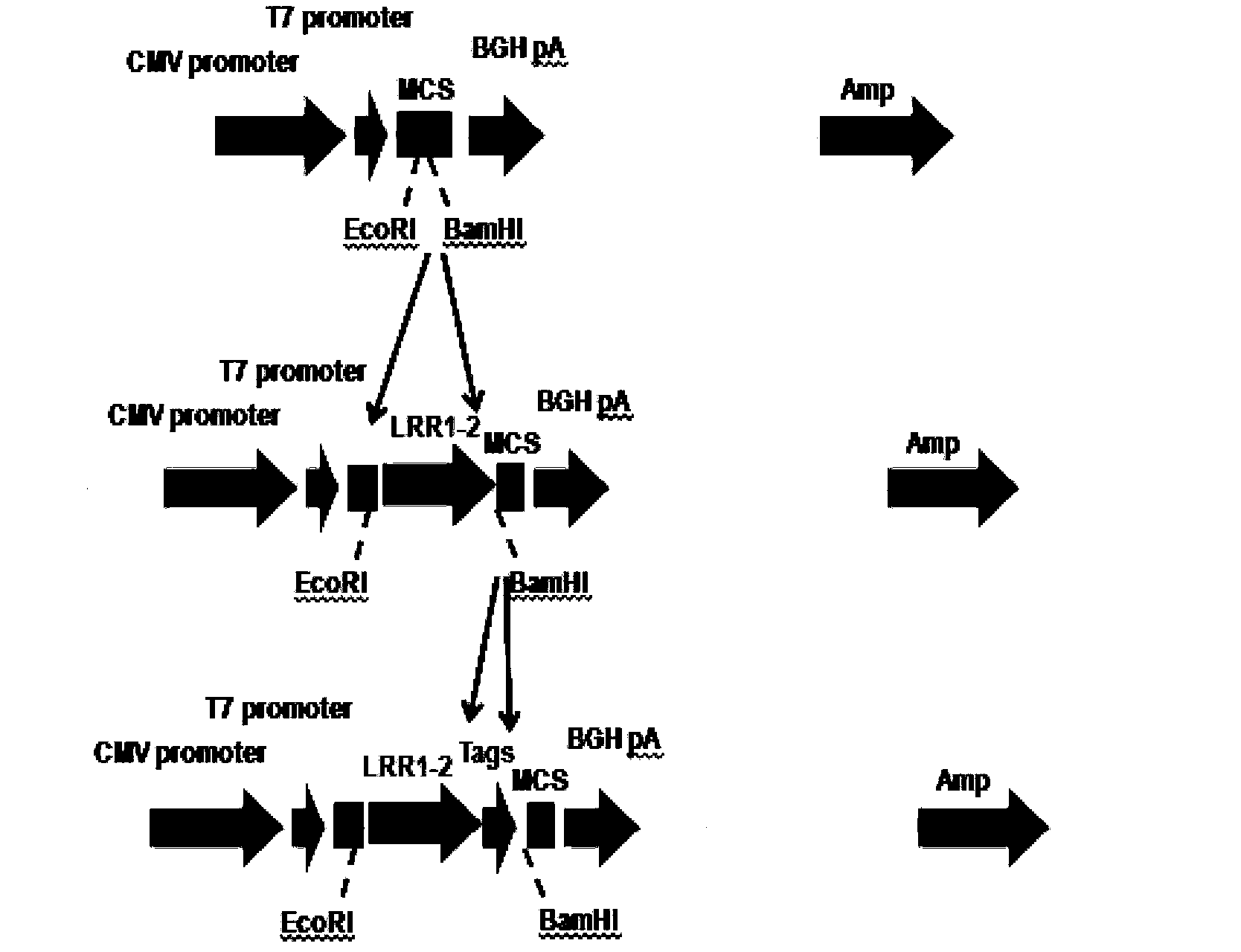
[0130] Example 1 Construction of pcDNA3.1(-) / Slit2LRR1-2 vector
[0131] Method: Use exonuclease method to construct plasmid. In the process of constructing the plasmid, two fragments (signal peptide sequence + target sequence, restriction site + tag protein sequence) were amplified by co-PCR, and the fragments were inserted twice. The primers used are Primer1 and Primer2 respectively.
[0132] Specific steps of exonuclease method:
[0133] 1. Prepare the reaction system: 50ng each of the vector fragment (recovered by the gel after digestion with BamHI and determine the concentration) and PCR segment (recovered by the gel after PCR and determine the concentration), 1ul10x exonuclease buffer, supplemented with ddH 2 O to 10ul;
[0134] 2. Place on ice for 5 minutes, add 1ul of Exonuclease III (Takara) diluted to 20U / ul, mix well and place on ice for 60 minutes;
[0135] 3. Add 1ul0.5M EDTA (pH8.0) solution to stop the reaction, and then water bath at 65℃ for 5min;
[0136] 4. Place on i...
Embodiment 2
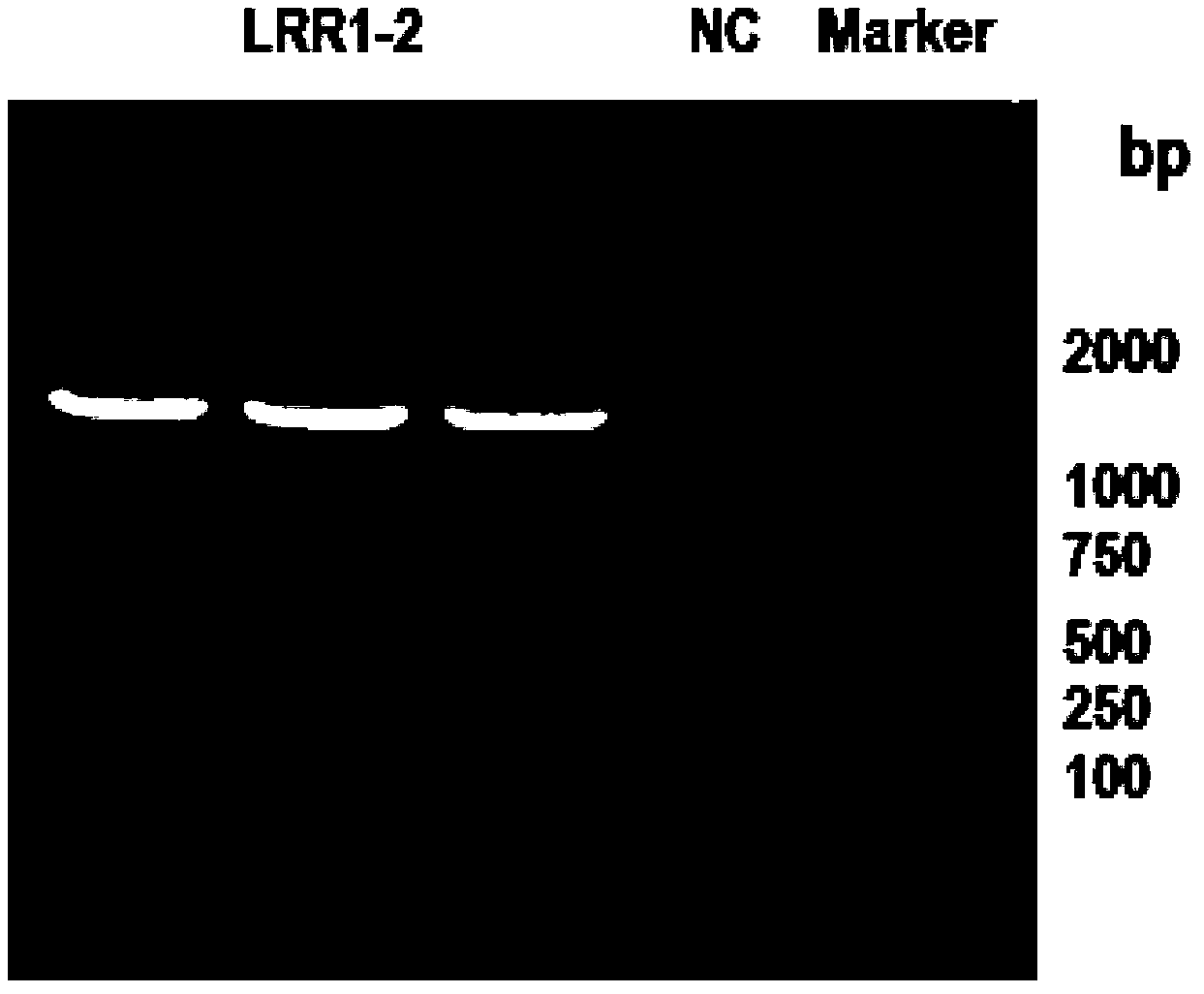
[0144] Example 2 Successful expression of pcDNA3.1(-) / Slit2LRR1-2
[0145] Method: In order to detect whether the constructed plasmid was successfully expressed, the plasmid was transfected into AD293 cells, and then the Western Blot was made with the tag protein 8×His to detect whether the fusion protein was successfully expressed.
[0146] Materials: High glucose DMEM medium and fetal bovine serum were purchased from HyClone; the transfection reagent Magetran was purchased from Origene; AD293 cells were cultured in High glucose DMEM medium containing 10% fetal bovine serum. The inverted microscope is a product of Olympus, and the fluorescent inverted microscope is a product of Nikon.
[0147] step:
[0148] 2.1. Resuscitate and culture the frozen cells, pass them 3 to 4 times to make the cells reach a good growth state, and carry out the plating test. Magetran reagent transfects adherent cells.
[0149] 2.2. Inoculate AD293 cells to a 6-well plate and culture it with High glucose DM...
Embodiment 3
[0152] Example 3 Test of expression level
[0153] A purified HisGFP protein of known concentration (20μg / ml) was used as a control, and the protein expression level was determined by the method of Western Blot.
[0154] The specific method is the same as in Example 2. The cells are transfected first, and then Western Blot is performed. The difference is that when running SDS-PAGE electrophoresis, a certain known amount of HisGFP protein is added as a positive control.
[0155] After the result of exposure is obtained, the expression of protein is calculated by gray analysis of the band.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com