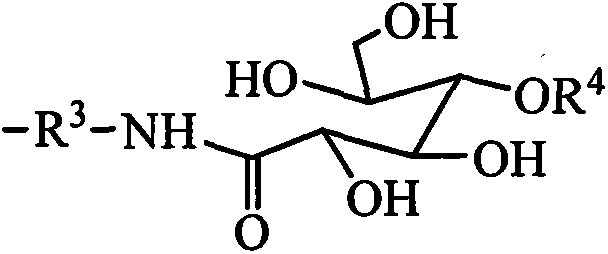
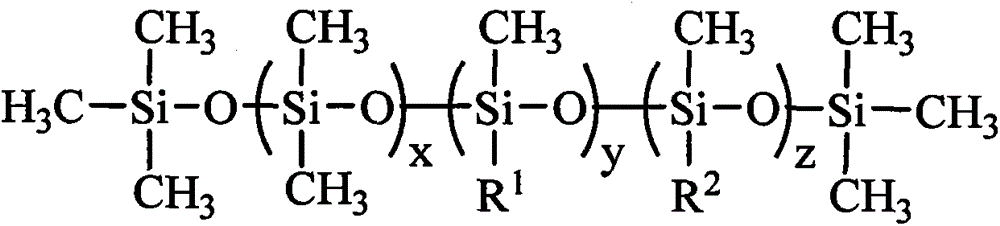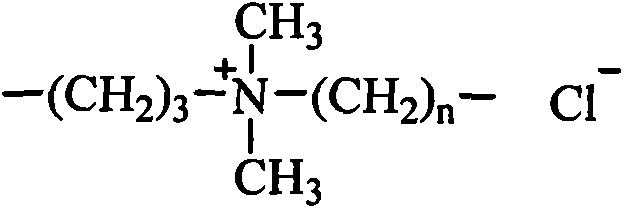Organosilicon quaternary ammonium salt containing alkyl group and glycosylamide group, and preparation method thereof
A technology of organosilicon quaternary ammonium salt and dimethylaminoalkyl sugar amide, which is applied in the field of organosilicon quaternary ammonium salt containing alkyl and sugar amide groups and the preparation method, and can solve the problem of poor adsorption capacity and poor alkane/water system and other problems, to achieve the effect of strong adsorption capacity and good emulsifying capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Add 0.88kg of N,N-dimethylethylenediamine and 1.78kg of gluconolactone into the reactor 1, using methanol as a solvent, heating to 60°C, and reacting for 8 hours. The solvent methanol is evaporated to obtain N,N- Dimethyl ethyl glucamide. Add 1.83 kg of chloropropyl dimethoxymethyl silane, 2.66 kg of N,N-dimethyl ethyl glucamide, and 0.003 kg of potassium iodide into the reactor 2, using isopropanol as a solvent, and heating to 60°C, After reacting for 20 hours, the solvent is distilled off, and the sugar amide modified quaternary ammonium salt is obtained by extraction. Add 1.62kg of hexamethyldisiloxane, 8.88kg of hexamethylcyclotrisiloxane, 1.32kg of hexadecyldimethoxymethylsilane, and sugar amide modified quaternary ammonium salt into the reactor 3. 2.69kg, 21.72g tetramethylammonium hydroxide, heated to dissolve, the temperature was controlled at 100°C, after 4 hours of reaction, heated to 130°C to deactivate the catalyst, and evaporated under reduced pressure to r...
Embodiment 2
[0037] Add 1.02kg of N,N-dimethylpropanediamine and 1.96kg of gluconic acid to the reactor 1, using ethanol as a solvent, heating to 70°C, and reacting for 8 hours. The solvent ethanol is evaporated to obtain N,N-dimethyl Propyl glucamide. Add 3.66 kg of chloropropyl dimethoxymethyl silane, 2.80 kg of N,N-dimethyl propyl glucamide, and 0.09 kg of sodium iodide into the reactor 2, using isopropanol as a solvent, and heating to 70 After reacting at ℃ for 24 hours, the solvent and excess reactants were evaporated under reduced pressure, and the sugar amide modified quaternary ammonium salt was obtained by extraction. Add 1.62kg of hexamethyldisiloxane, 8.88kg of octamethylcyclotetrasiloxane, 1.09kg of octyldimethoxymethylsilane, and 4.63kg of sugar amide modified quaternary ammonium salt into the reactor 3. kg, potassium hydroxide 36.40g, heated to dissolve, the temperature is controlled at 120°C, after 5 hours of reaction, add acetic acid to neutralize the catalyst to deactivate...
Embodiment 3
[0039] Add 1.16 kg of N,N-dimethylbutanediamine and 1.78 kg of mannonic acid lactone into the reactor 1, using methanol as a solvent, heating to 80°C, and reacting for 10 hours. The solvent methanol is evaporated to obtain N, N -Dimethyl butyl mannose amide. Add 5.49 kg of chloropropyl dimethoxymethyl silane, 2.94 kg of N,N-dimethyl butyl mannose amide, and 0.26 kg of potassium iodide into the reactor 2, using n-butanol as a solvent, and heating to 80°C, After reacting for 24 hours, the solvent and excess reactants are evaporated under reduced pressure, and the sugar amide modified quaternary ammonium salt is obtained by extraction. Add 1.62kg of hexamethyldisiloxane, 4.44kg of hexamethylcyclotrisiloxane, 1.37kg of lauryldimethoxymethylsilane, and 9.54kg of sugar amide modified quaternary ammonium salt into the reactor 3 , Tetrabutyl phosphonium hydroxide 1.52g, heated to dissolve, the temperature was controlled at 80°C, after 4 hours of reaction, heated to 110°C to deactivate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com