Mycoplasma arthritis arginine deiminase mutant and its application
An arginine deiminase and amino acid technology, which is applied in the pharmaceutical field of biologically active proteins, can solve the problems of difficult industrial production of arginine deiminase, and achieve the effects of improving the renaturation efficiency and increasing the expression amount.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The design of the directed mutation of the arginine deiminase gene of embodiment 1 mycoplasma arthritis
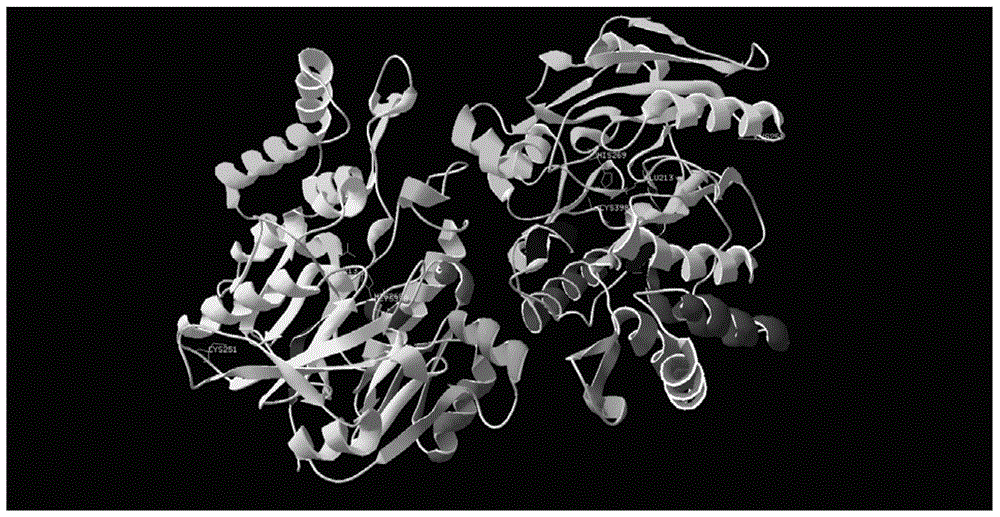
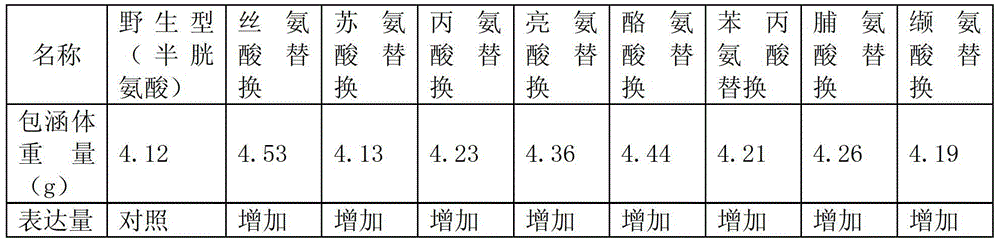
[0046] Based on the coding nucleotide sequence of the arginine deiminase of ATCC14152 strain Mycoplasma arthritidis (Mycoplasma arthritidis) in U.S. Patent US5804183, simulate the arginine deiminase in Mycoplasma arthritidis with PyMOL0.99rc6 software 3D structure (see figure 1 ), it can be seen that the cysteine group C251 is far away from the active center of arginine deiminase, and other amino acids (serine, threonine, glycine, alanine, leucine, isoleucine, tyrosine acid, phenylalanine, proline or valine) to replace the cysteine group C251, and 10 mutants were designed. Three-dimensional structure analysis showed that these mutations did not significantly change the three-dimensional structure of arginine deiminase.
Embodiment 2
[0047] Example 2 Directed Mutation and Recombinant Expression of Mutant Arginine Deiminase Gene
[0048] Based on the nucleotide sequence encoding arginine deiminase from ATCC14152 strain Mycoplasma arthritis described in U.S. Patent No. 5,804,183, according to Escherichia coli encoding serine, threonine, glycine, alanine, leucine, Different codons for isoleucine, tyrosine, phenylalanine, proline and valine, design mutated nucleotide sequences, and entrust the gene company to synthesize nucleotides with the original enzyme sequence and mutant sequence .
[0049] The proenzyme gene and the arginine deiminase gene produced by directed mutation were inserted behind the T7 promoter of the plasmid pET-27b(+). Then transform into competent Escherichia coli BL21 cells (Escherichia coli B series 834 strain, Escherichia coli834strain). The DNA sequence of the targeted mutation gene was confirmed by nucleotide sequence analysis as shown in SEQ ID NO.3.
[0050] The wild-type proenzym...
Embodiment 3
[0053] Refolding and purification of embodiment 3 directed mutation arginine deiminase
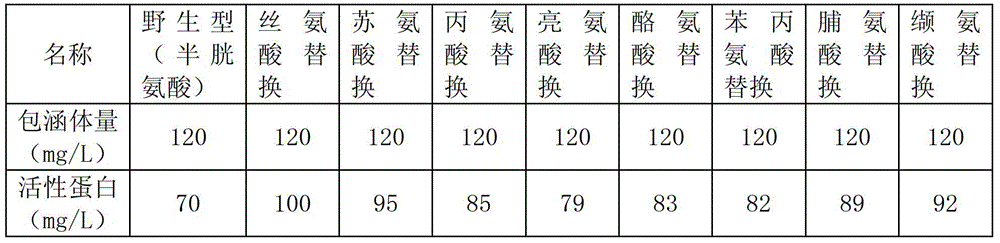
[0054] The Escherichia coli cells harvested in Example 2 were centrifuged to obtain a bacterial cell precipitate. Suspend 10 g of bacterial sediment in 100 ml of 10 mM sodium phosphate buffer (pH 7.0). After sonicating the cells, 4% TritonX-100 was added and stirred at 4°C for 30 min. Inclusion bodies were obtained by centrifugation at 13000g for 30 minutes. Then the proenzyme inclusion body was suspended in 10 ml of 50 mM Tris (pH 8.5) solution containing 6M guanidine hydrochloride and 10 mM dimercaptothreitol (DTT), and stirred at 4°C for 15 minutes. Finally, the solution was added to 1 liter of 10 mM sodium phosphate buffer (pH 7.0), stirred at 15°C for 90 hours, and 70 mg of purified arginine deiminase was obtained per liter of culture solution.
[0055] Suspend the inclusion body of directed mutation of arginine deiminase in 10 ml of 50 mM Tris (pH 8.5) solution containing 6M guani...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com