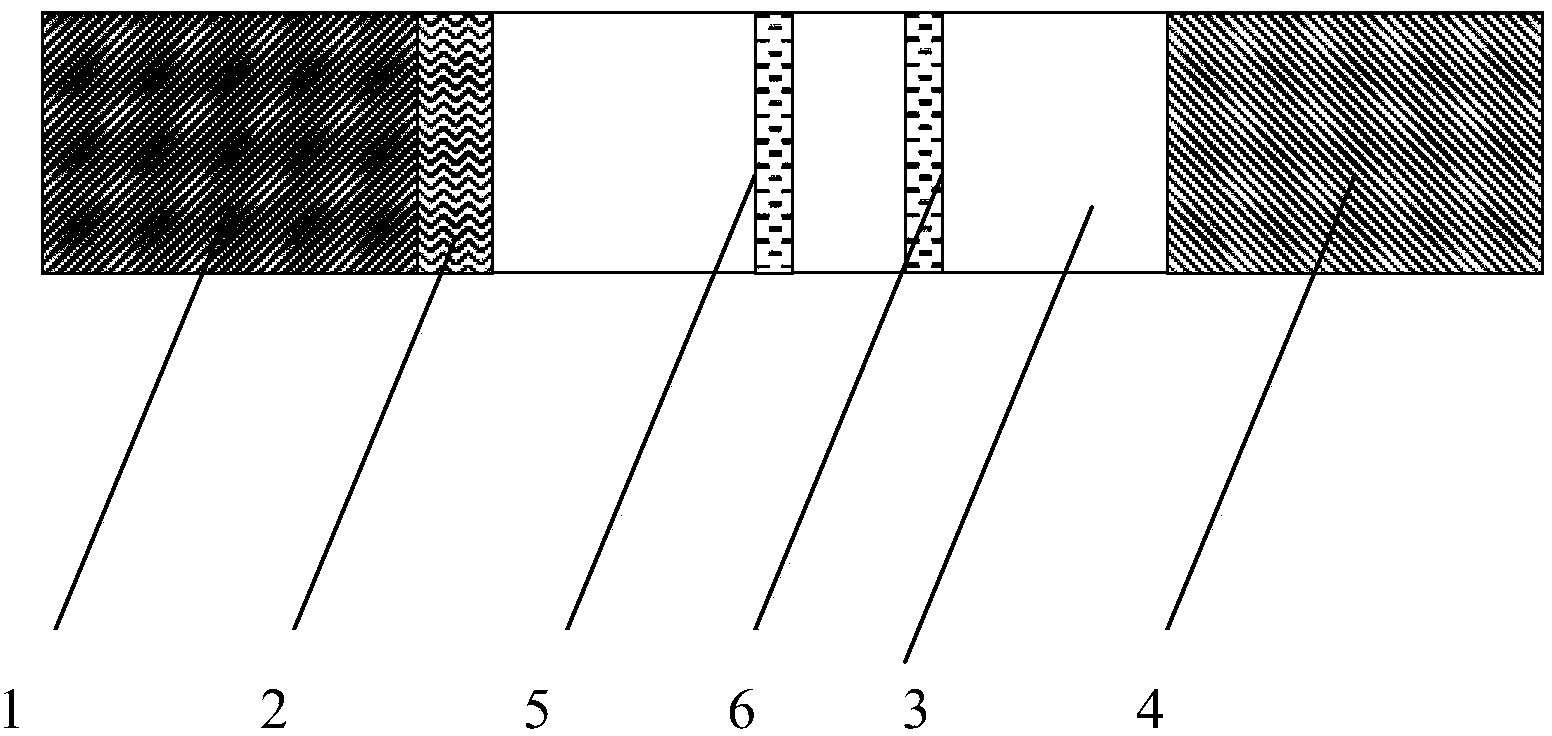
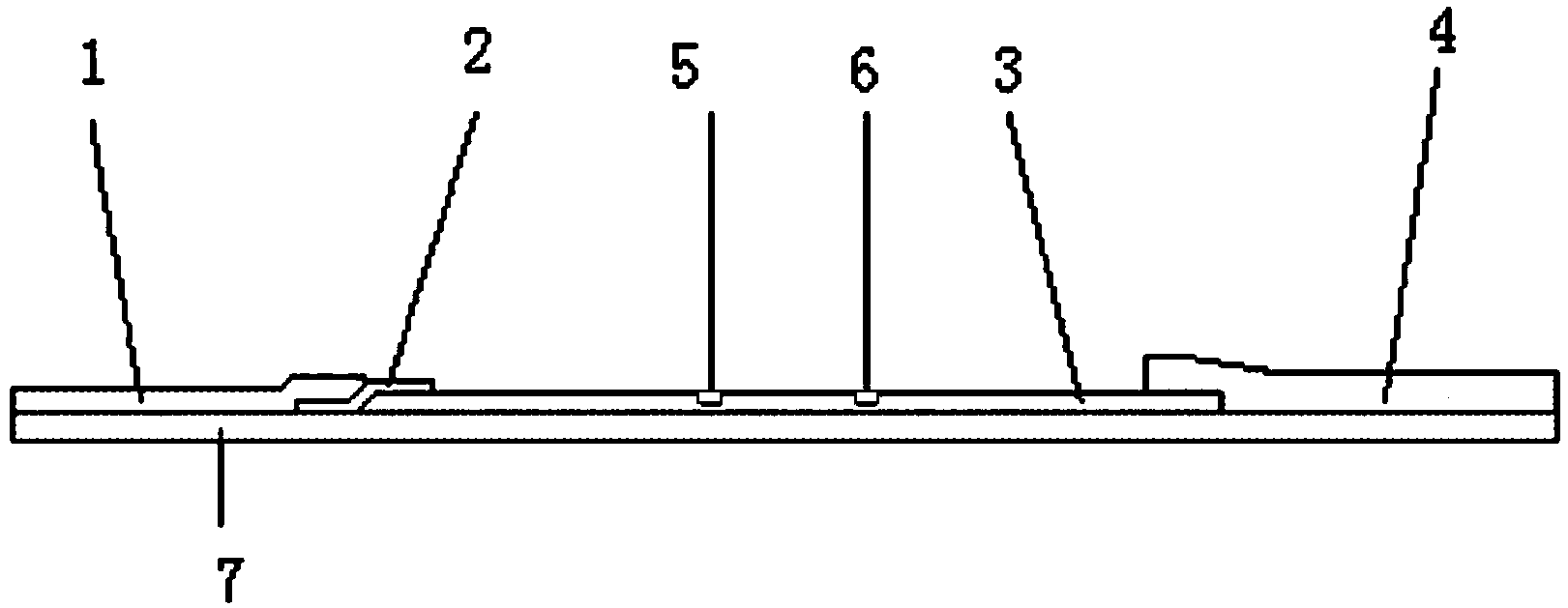
Immunochromatographic test strip for diagnosing kala-azar based on detection of circulating antigens
An immune chromatography and kala-azar technology, applied in the field of bioengineering, can solve the problems of low accuracy, time-consuming and labor-intensive diagnosis of kala-azar, and achieve the effects of easy industrial production, broad application prospects, high sensitivity and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Preparation of Leishmania donovani Amastigote Body Antigen
[0031] The amastigote antigen of Leishmania donovani was prepared as follows: take the proflagellates of Leishmania donovani 801 (MHOM / CN / / 801 / XJ) cultured in NNN medium at 22°C The body was inoculated in 199 medium (pH7.2-7.4) and expanded at 22°C. After the promastigotes reached a certain density, they were collected by centrifugation, and transferred to 199 medium (PH5.4) at 37°C for amastigote transformation. Centrifuge the transformed amastigotes at 3000g at 40C for 15 minutes to collect the amastigotes, discard the supernatant, and wash the pellet with PBS three times in the same way. In a water bath at 37°C, freeze and thaw repeatedly 5 times, then ultrasonically pulverize 3 times in an ice bath, centrifuge at 18000g, 4°C for 20min, and the supernatant is the soluble antigen. BALB / c mice were immunized with the antigen prepared by the above method, splenocytes from the immunized mice were fu...
Embodiment 2
[0034] Example 2 Preparation of monoclonal antibody against Leishmania donovani amastigote antigen
[0035] Immunization: Each BALB / c mouse was injected intraperitoneally with 100 μg of the suspension of the above-mentioned purified Leishmania donovani amastigote soluble antigen + Freund’s complete adjuvant for the first time, and then injected with 100 μg of purified Leishmania donovani every 1 month Protozoan amastigote soluble antigen + Freund's incomplete adjuvant suspension once, a total of 2 times, and 3 days before the mouse was killed and the spleen was taken for cell fusion, the antigen was directly injected through the tail vein to boost the immunization once.
[0036] Production and screening of hybridoma cells: fusion of SP2 / 0 tumor cells and splenocytes of immunized mice and cloning of hybridoma cells were carried out according to conventional methods in the art. The above-mentioned purified Leishmania donovani amastigote soluble antigen and glutathione-S-transfer...
Embodiment 3
[0044] Example 3 Preparation of immunochromatographic test strips for diagnosis of kala-azar
[0045] 1. The monoclonal antibody against the soluble antigen of Leishmania donovani was prepared from Example 2.
[0046] 2. Goat anti-mouse IgG was purchased.
[0047] 3. Preparation of immunocolloidal gold probes and gold standard pads
[0048] The monoclonal antibody colloidal gold probe and the gold standard pad of the soluble antigen of Leishmania donovani were prepared by the following method:
[0049] 1) Prepare colloidal gold particles by citrate reduction method, the specific method is: HAuCl 4 (Shanghai trial brand was purchased from Shanghai Sinopharm Chemical Reagent Co., Ltd.) to prepare 0.01% aqueous solution, take 100mL and heat to boiling, accurately add 1.6mL of 1% trisodium citrate aqueous solution under stirring, until the color of the liquid stabilizes into wine red, A colloidal gold solution is obtained.
[0050] 2) Determine the saturation concentration of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com