Liposomal corticosteroids for treatment of inflammatory disorders in humans
A technology for inflammatory diseases and corticosteroids, used in liposome delivery, urinary system diseases, anti-inflammatory agents, etc., can solve the problems of increasing medical cost burden and high cost of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
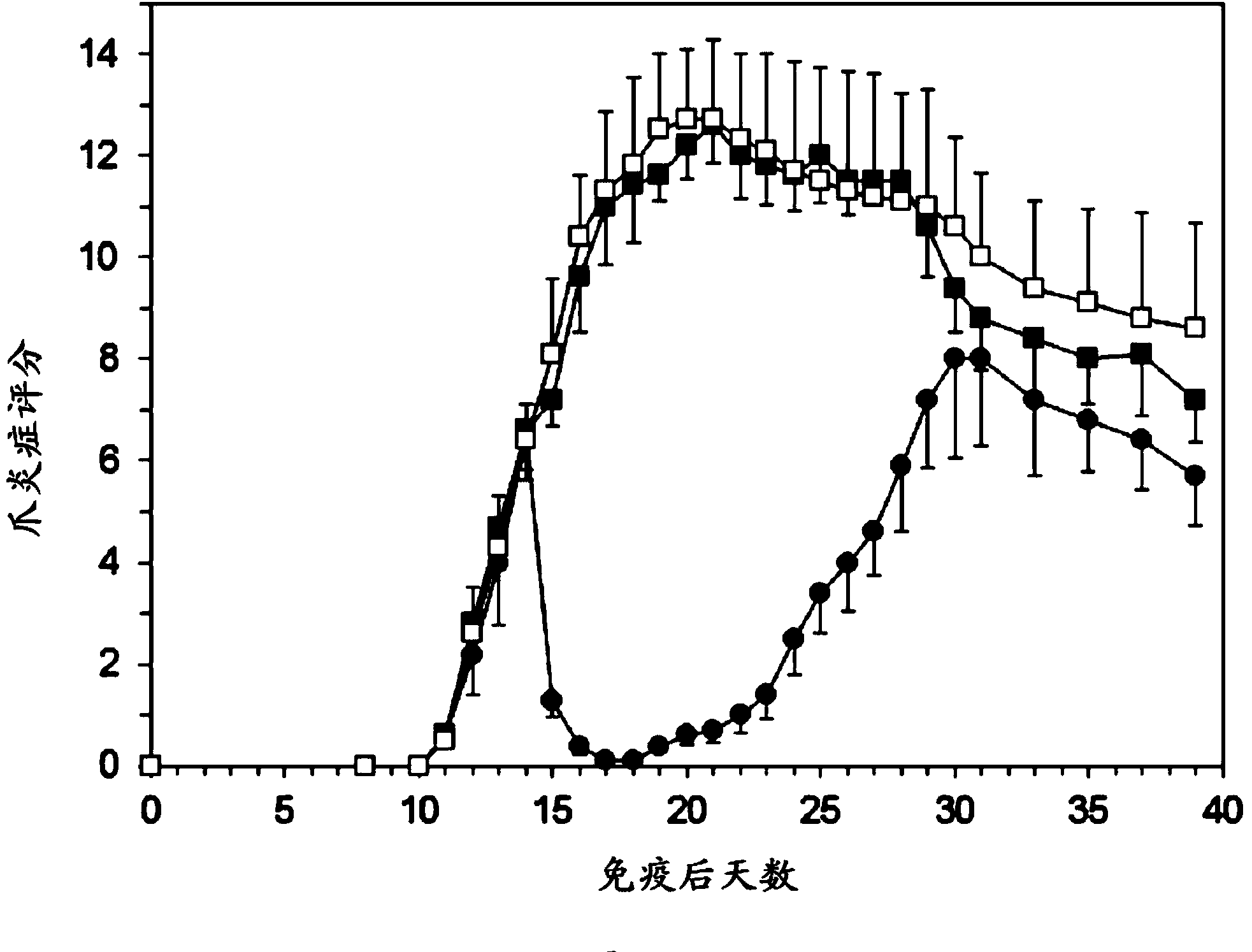
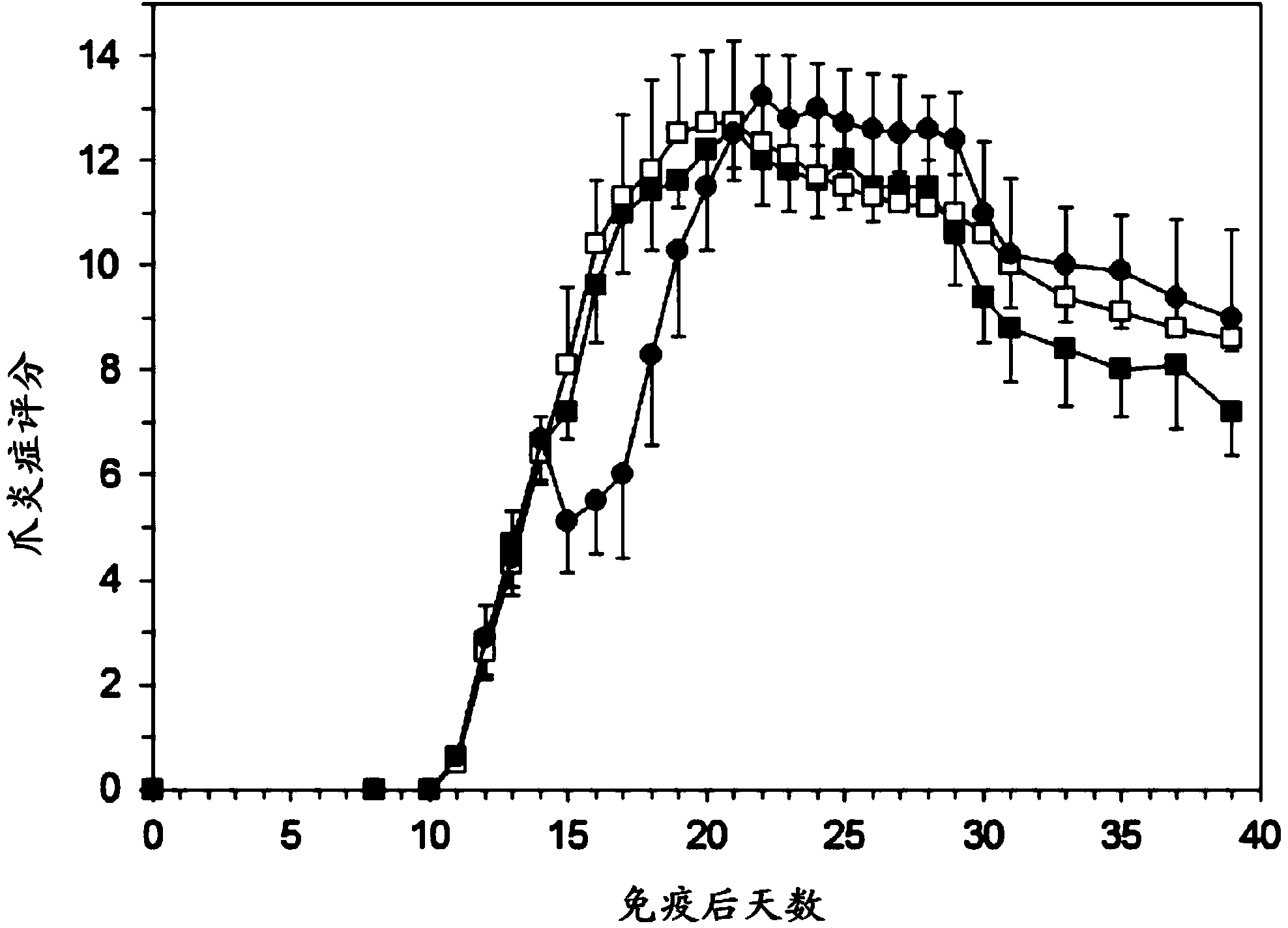
[0069] Example 1: Experimental Arthritis Study in Rats
[0070] preparation
[0071] PEG liposomes containing prednisolone phosphate are composed of 750 mg of dipalmitoylphosphatidylcholine (DPPC), 250.8 mg of cholesterol and 267.6 mg of PEG-distearoylphosphatidylethanolamine (PEG-DSPE) . The components were weighed and mixed in a 100ml round bottom flask. Lipids were dissolved in about 30 ml of ethanol and then evaporated to dryness under vacuum in a rotary evaporator at 40° C. within 1 hour. Weigh 1200 mg of prednisolone disodium phosphate and dissolve it in 12 ml of sterile water. This solution was added to the dried lipid film and shaken within one hour in the presence of glass beads to fully hydrate the lipid film. The liposome suspension was transferred to an extruder (Avestin, maximum volume 15 ml) and extruded under pressure using nitrogen gas using a polycarbonate filter with a pore size below 100 nm. Then, dialyze with sterile saline. The average particle size ...
Embodiment 2
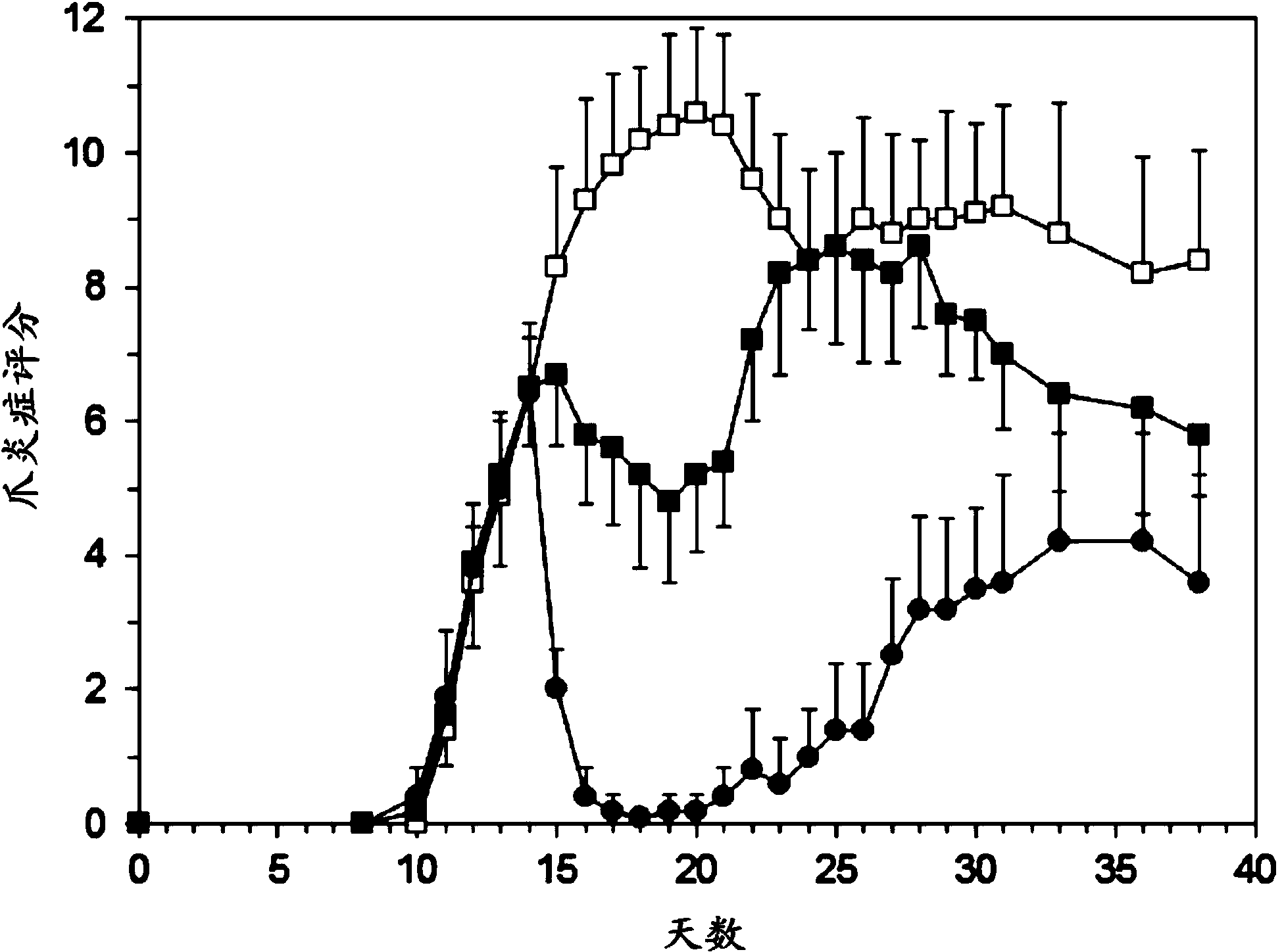
[0077] Example 2: Human RA Study
[0078] preparation
[0079]Prednisolone containing polyethylene glycol (PEG) consists of a lipid bilayer surrounding an aqueous compartment in which a water-soluble disodium phosphate derivative of prednisolone is encapsulated. Each mL formulation contains 1.5 mg / mL prednisolone sodium phosphate, 30 mg palmitoylphosphatidylcholine (DPPC), 9 mg distearoylphosphatidylethanolamine-PEG2000 (PEG-DSPE) and 8 mg steroid. Liposomes were dispersed in 10% sucrose, pH 7.4, buffered with phosphate buffer.
[0080] The formulation is prepared by mixing the lipid component with an aqueous solution of the corticosteroid, followed by repeated high shear homogenization to reduce the size of the vesicles formed. Unencapsulated corticosteroids were removed by tangential flow filtration. Sterilize by dead-end filtration using a 0.2 micron filter.
[0081] The formulations were characterized and quality controlled by: particle size and polydispersity index (1...
Embodiment 3
[0095] Example 3: Human Inflammatory Bowel Disease Research
[0096] preparation
[0097] Prednisolone containing PEG liposomes was prepared as described in Example 2.
[0098] patient
[0099] Twenty patients with active ulcerative colitis (UC) between the ages of 18 and 75 years were selected during the 14-day screening phase according to the following main inclusion / exclusion criteria: age ≥18 years to 75 years, by Endoscopically evaluated and documented history of UC (at least 6 months), Mayo score ≥5 with a subscore of ≥2 for endoscopy and ≥1 for rectal bleeding, and stable medication (6 - MP / azathioprine, 5-ASA, MTX, biologics) and in good physical and mental health (except for the disease being studied) as determined by history and physical examination.
[0100] research proposal
[0101] When subjects were randomly assigned to the study product arm, 150 mg PEG liposomal prednisolone sodium phosphate (Nanocort) IV in 250 mL of normal saline was infused alone over a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com