Heavy-load valproic acid drug sustained release tablet and preparation method thereof
A technology for valproic acid and sustained-release tablets, which is applied in directions such as pharmaceutical formulations, drug combinations, and drug delivery, can solve the problems of complex preparation process, unstable release and high production cost, and achieves simple preparation process, convenient swallowing, Easy-to-operate effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Sodium valproate extended-release tablets:
[0049]
[0050] Tablet weight is 824mg, made into 1000 pieces
[0051] Preparation Process:
[0052] Sodium valproate and auxiliary materials are passed through an 80-mesh sieve, the molecular weight of chitosan is 400kDa, and the degree of deacetylation is 50%, and each component except the lubricant magnesium stearate and the anti-adhesive micropowder silica gel is mixed in equal amounts Evenly, add an appropriate amount of 70% ethanol solution as a binder, make a soft material, granulate, dry at 55°C, granulate, add lubricant magnesium stearate and anti-sticking agent micro-powder silica gel to mix evenly, place Compressed into tablets in a tablet machine.
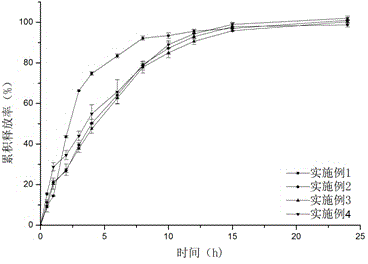
[0053] The self-made sustained-release tablet was placed in the dissolution vessel for dissolution test, and the in vitro release results were as follows: figure 1 shown.
Embodiment 2
[0055] Compound Sodium Valproate Sustained Release Tablets:
[0056]
[0057]
[0058] Tablet weight is 882mg, made into 1000 pieces
[0059] Preparation Process:
[0060] The sodium valproate and auxiliary materials are passed through an 80-mesh sieve, and the liquid valproic acid is adsorbed by adsorbent micropowder silica gel, so that it is adsorbed into a uniform powder, and then chitosan (400kDa, deacetylation degree 75%), alginic acid Add sodium, sodium carboxymethylcellulose, and sodium valproate in sequence, and mix well. Add an appropriate amount of 70% ethanol solution as a binder, make soft materials, granulate, dry at 55°C, granulate, add lubricant magnesium stearate and anti-sticking agent micro-powder silica gel, mix well, place Compressed into tablets in a tablet machine.
[0061] The self-made sustained-release tablet was placed in the dissolution vessel for dissolution test, and the in vitro release results were as follows: figure 1 shown.
Embodiment 3
[0063] Compound Sodium Valproate Sustained Release Tablets
[0064]
[0065] The tablet weight is 902mg to make 1000 tablets
[0066] Preparation Process:
[0067] The sodium valproate and auxiliary materials are passed through 80 mesh sieves, and the liquid valproic acid is adsorbed by adsorbent pregelatinized starch, so that it is absorbed into a uniform powder, and then chitosan (200kDa, deacetylation degree 75%), Add sodium alginate, carrageenan, and sodium valproate in sequence, and mix well. Add an appropriate amount of 50% ethanol solution as a binder, make soft materials, granulate, dry at 80°C, granulate, add lubricant magnesium stearate and anti-sticking agent micro-powder silica gel, mix well, place Compressed into tablets in a tablet machine.
[0068] The self-made sustained-release tablet was placed in the dissolution vessel for dissolution test, and the in vitro release results were as follows: figure 1 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com