Canagliflozin new preparation method
A technology of canagliflozin and a synthesis method, applied in the field of pharmacy, can solve problems such as excessive reaction time, large amount of methanesulfonic acid, corrosion, etc., and achieves the advantages of reducing the amount of methanesulfonic acid, being extremely easy to recover and applying, and reducing costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
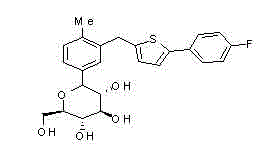
[0039] 1-[1-Hydroxy-2,3,4,6-tetra-O-(trimethylsilyl)-β-D-glucopyranosyl]-4-methyl-3-[5-( Synthesis of 4-fluorophenyl)-2-thienylmethyl]benzene
[0040] Add compound 7 (84.00 g, 0.23 mol) into a 3000 mL dry three-neck flask, install a mechanical stirring paddle, install a tee on one side and connect it to the nitrogen bag, and install a constant pressure dropping funnel on the other side (sealed with a flip plug) ), fix the device. Nitrogen was exchanged three times, then 800 mL of toluene and 800 mL of THF were added, stirred to dissolve, cooled in liquid nitrogen-acetone, and at the same time, 90 mL of n-BuLi (0.24 mol, 2.7 M n-hexane alkane solution). After the temperature dropped below -78 °C, continue to stir for 20 min, then slowly add n-BuLi cyclohexane solution dropwise into the constant pressure dropping funnel, and the dropwise addition was completed within 30 min. Stirring was continued at this temperature for 30 min. Then the dropping funnel was replaced with a c...
Embodiment 2
[0043] 1-[1-Hydroxy-2,3,4,6-tetra-O-(trimethylsilyl)-β-D-glucopyranosyl]-4-methyl-3-[5-( Synthesis of 4-fluorophenyl)-2-thienylmethyl]benzene
[0044] Add the compound 2-(4-fluorophenyl)-5-(5-iodo-2-methylphenyl)methylthiophene (70.40 g, 0.17 mol) into a 3000 mL dry three-necked flask, install a mechanical stirring paddle , One side is connected with a three-way connection with the nitrogen bag, and the other side is installed with a constant pressure dropping funnel (sealed by turning over the plug), and the device is fixed. Nitrogen was exchanged three times, then 700 mL of toluene and 700 mL of THF were added, stirred to dissolve, cooled in liquid nitrogen-acetone, and 114 mL of n-BuLi (0.18 mol, 1.6 M n-hexane solution). After the temperature dropped below -78 °C, continue to stir for 20 min, then slowly add n-BuLi cyclohexane solution dropwise into the constant pressure dropping funnel, and the dropwise addition was completed within 30 min. Stirring was continued at th...
Embodiment 3
[0047] Synthesis of 1-(1-methoxy-2-β-D-glucopyranosyl)-4-methyl-3-[5-(4-fluorophenyl)-2-thienylmethyl]benzene
[0048] Dissolve the target product in Example 1 with 1000 ml of methanol, add 0.5 ml of methanesulfonic acid, react at 40°C for 2 h, distill off the solvent under reduced pressure, add 1000 ml of ethyl acetate, transfer to a separatory funnel, and successively saturated carbonic acid Wash with sodium hydrogen and saturated brine, dry the organic phase with anhydrous sodium sulfate, evaporate the solvent on a rotary evaporator to obtain a raw brown solid, add 300 ml of toluene to mechanically stir to dissolve, add 1200 ml of cyclohexane, and precipitate an off-white solid product (90.48 g, 82.0% yield based on 2-(2-methyl-5-bromobenzyl)-5-(4-fluorophenyl)thiophene).
[0049] ESI-MS m / z 460.2 (M+NH4-MeOH), 443.1 (M+H-MeOH). 1H NMR (400MHz, DMSO-d6): δ 2.26 (s, 3H), 2.92 (d, 1H, J= 8.4Hz), 2.96 (s, 3H), 3.22 (t, 1H, J=9.0Hz), 3.36-3.40 (m, 1H), 3.52-3.61 (m, 2H), 3.76 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com