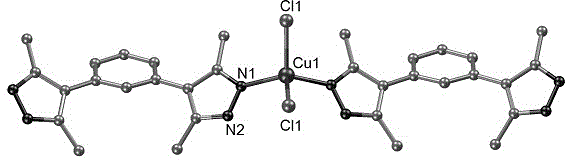
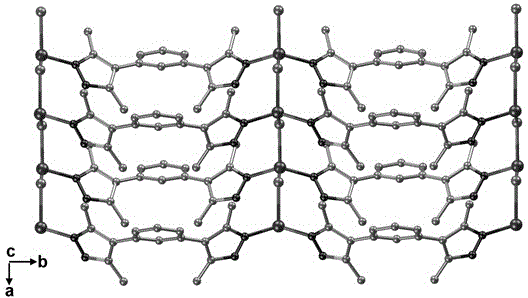
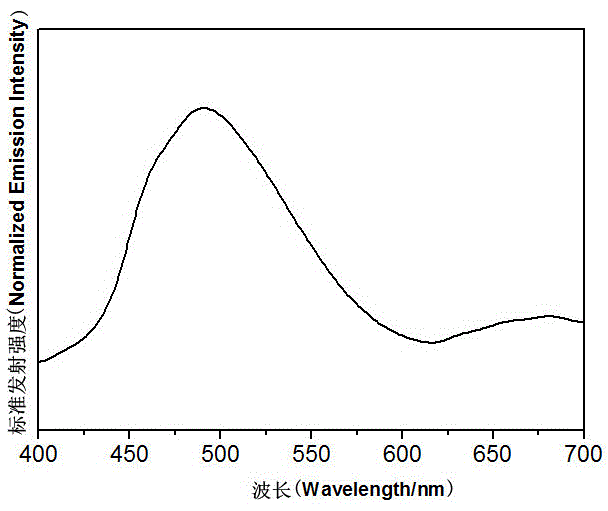
Two-dimensional coordination polymer and preparation method thereof
A technology of coordination polymer, m-phenylpyrazole, applied in copper organic compounds, chemical instruments and methods, luminescent materials, etc., to achieve the effects of easy preparation, excellent luminescent properties, and fewer material defects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] CuCl 2 (0.1mmol), m-phenylpyrazole (0.05mmol) and ethanol (5mL) were added to a polytetrafluoroethylene autoclave, stirred at room temperature for 10min, then heated to 130°C at a rate of 5°C per hour, kept for 60h, and directly Cool to room temperature. Filtration and washing yielded a coordination polymer with a helical structure, with a yield of about 83%. Infrared spectral data (KBr, cm -1 ):3443 (m), 3236 (s), 2912 (w), 1582 (s), 1516 (s), 1439 (s), 1234 (m), 798 (s), 703 (s), 650 (s ), 609 (m).
[0026] Then the above-mentioned coordination polymer with helical structure was characterized
[0027] The crystal X-ray diffraction data were determined by Burkcer Smart CCD single crystal diffractometer. MoKa radiation (λ=0.71073 ?), graphite monochromator, data collected in ω-scan mode, with Lp factor correction and empirical absorption correction. First use the direct method to determine the position of metal atoms and some other non-hydrogen atoms, then use the...
Embodiment 2
[0033] CuCl 2 (0.12mmol), m-phenylpyrazole (0.05mmol) and ethanol (5mL) were added into a polytetrafluoroethylene autoclave, stirred at room temperature for 10min, then heated to 140°C at a rate of 4°C per hour, kept for 48h, and directly Cool to room temperature. Filtration and washing yielded a helical coordination polymer with a yield of about 78.3%.
Embodiment 3
[0035] CuCl 2 (0.1mmol), m-phenylpyrazole (0.06mmol) and ethanol (5mL) were added to a polytetrafluoroethylene autoclave, stirred at room temperature for 10min, then heated to 150°C at a rate of 3°C per hour, kept for 26h, and directly Cool to room temperature. Filtration and washing yielded a coordination polymer with a helical structure, with a yield of about 79.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com