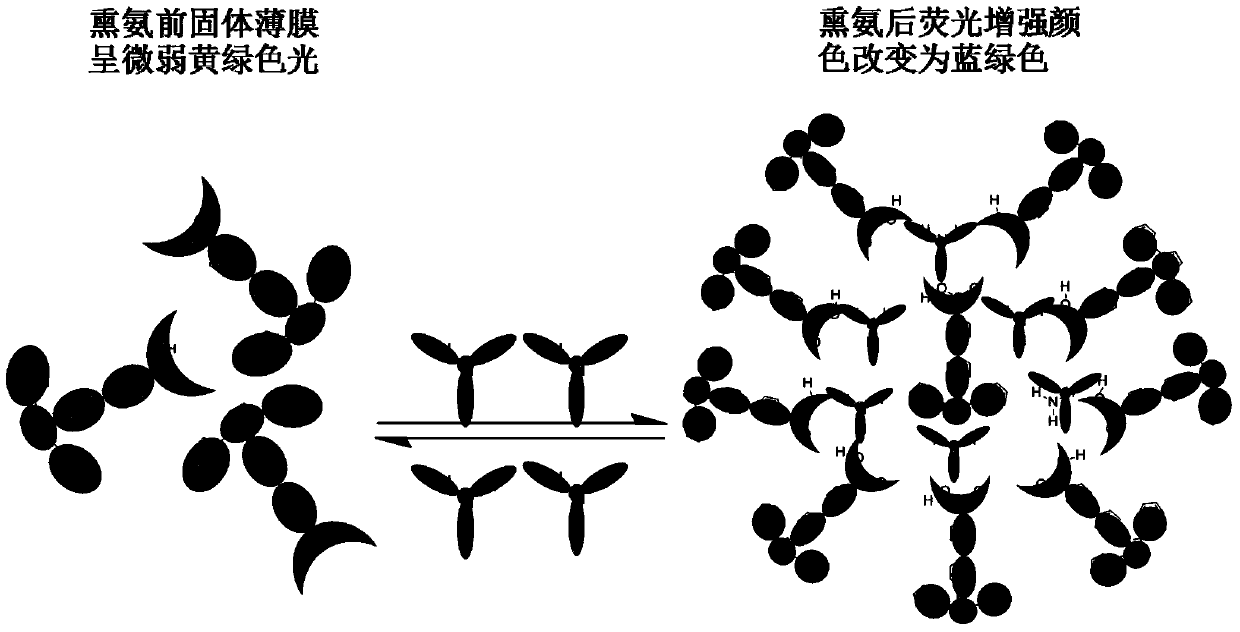
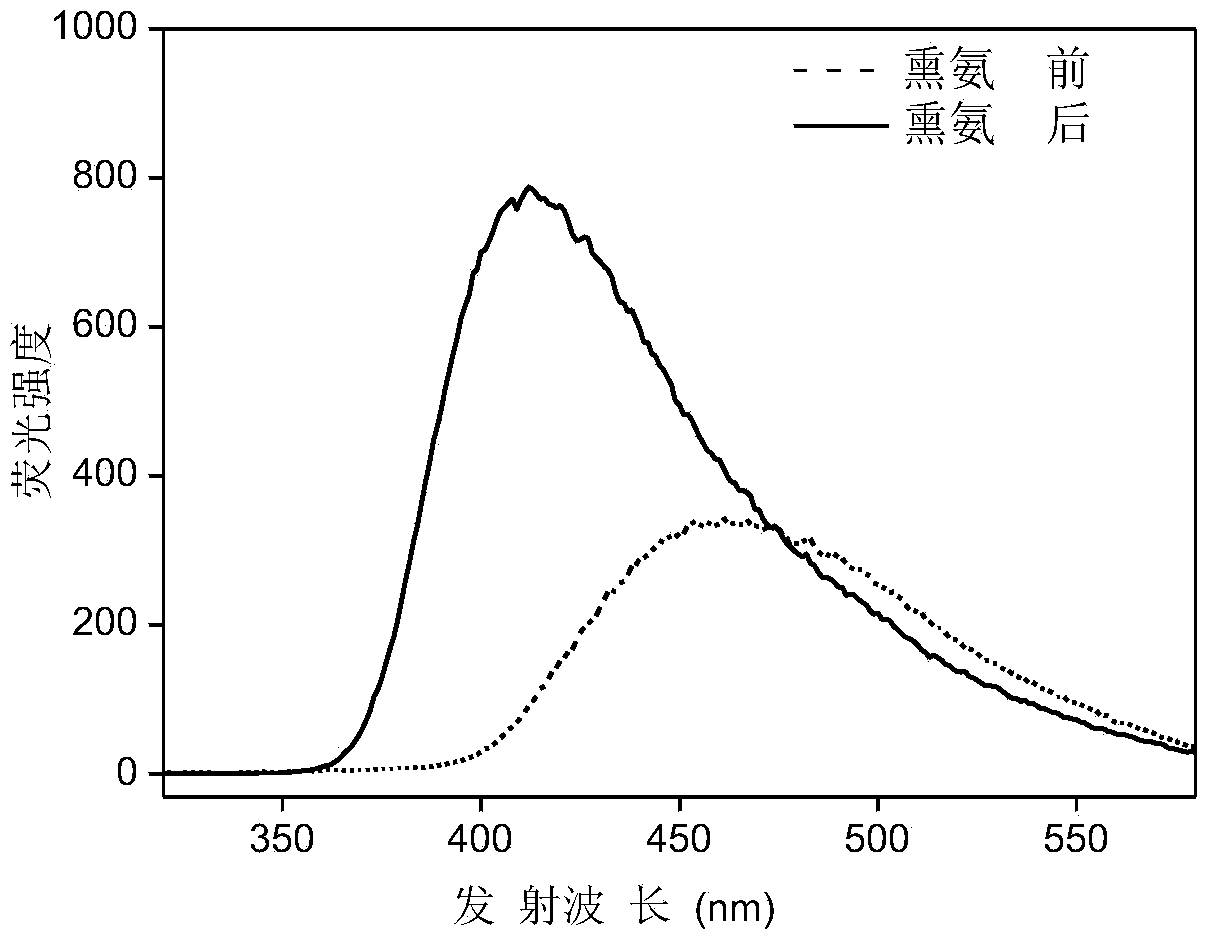
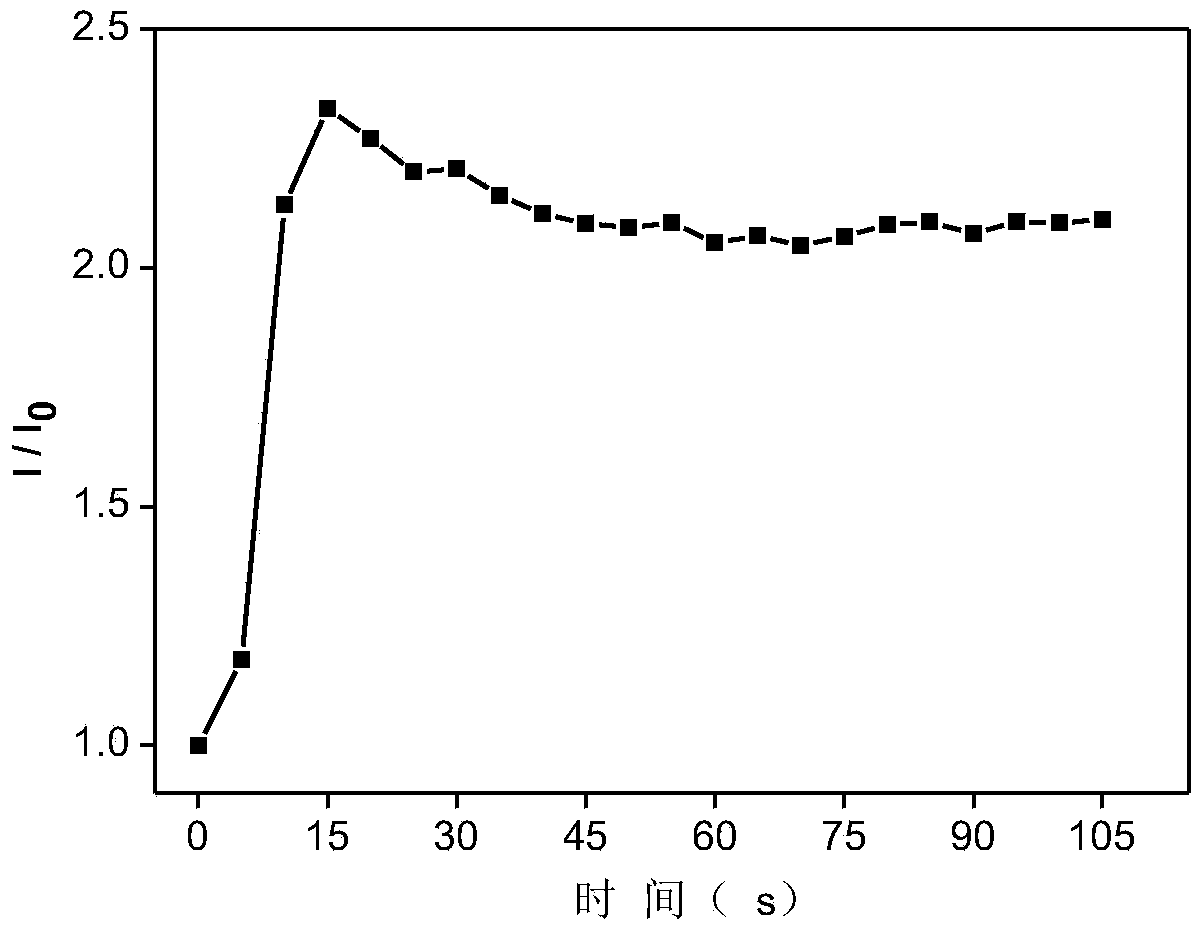
Fluorescent material capable of visual detection of ammonia gas as well as preparation method and application of fluorescent material
A technology of fluorescent material and ammonia gas, which is applied in the field of fluorescent sensors, can solve the problems of slow response time and difficulty in carrying around, and achieve the effects of convenient detection and operation, simple preparation method and remarkable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] (1) Preparation of CMP-TPP
[0053] Weigh 2.05g (5.50mmol) of (1-p-bromophenyl-2,5-diphenyl)pyrrole, 0.99g (5.48mmol) of 4-methoxycarbonylphenylboronic acid, 0.25g (0.14mmol) of tetrakis (triphenyl) Phenylphosphine) palladium, and 2.01g (18.97mmol) of anhydrous sodium carbonate were added in a 500mL round bottom flask, and the bottle mouth was sealed with a rubber stopper. Vacuumize - fill with nitrogen 3 times to remove all the oxygen in the bottle.
[0054] Inject 40mL of methanol and 100mL of toluene into the bottle with a syringe (the methanol and toluene were passed through nitrogen to remove oxygen in advance), and stirred at room temperature for 30min to fully mix and dissolve the substances in the system. Heated to 90°C, reacted for 24h, and ended the reaction.
[0055] The toluene was spin-dried, and the resulting solid was dissolved in dichloromethane (100 mL), washed with a 5% HCl solution of dilute hydrochloric acid (500 mL) to remove Na 2 CO 3 . Separa...
Embodiment 2
[0078] (1) Preparation of CMP-TPP
[0079] Weigh 2.05g (5.50mmol) of (1-p-bromophenyl-2,5-diphenyl)pyrrole, 0.99g (5.48mmol) of 4-methoxycarbonylphenylboronic acid, 0.25g (0.14mmol) of tetrakis (triphenyl) Phenylphosphine) palladium, and 2.01g (18.97mmol) of anhydrous sodium carbonate were added in a 500mL round bottom flask, and the bottle mouth was sealed with a rubber stopper. Vacuumize - fill with nitrogen 3 times to remove all the oxygen in the bottle.
[0080] Inject 40mL of methanol and 100mL of toluene into the bottle with a syringe (the methanol and toluene were passed through nitrogen to remove oxygen in advance), and stirred at room temperature for 30min to fully mix and dissolve the substances in the system. Heated to 100°C, reacted for 18h, and ended the reaction.
[0081] The toluene was spin-dried, and the resulting solid was dissolved in dichloromethane (100 mL), washed with a 5% HCl solution of dilute hydrochloric acid (500 mL) to remove Na 2 CO 3 . Separ...
Embodiment 3
[0104] (1) Preparation of CMP-TPP
[0105] Weigh 2.05g (5.50mmol) of (1-p-bromophenyl-2,5-diphenyl)pyrrole, 0.99g (5.48mmol) of 4-methoxycarbonylphenylboronic acid, 0.25g (0.14mmol) of tetrakis (triphenyl) Phenylphosphine) palladium, and 2.01g (18.97mmol) of anhydrous sodium carbonate were added in a 500mL round bottom flask, and the bottle mouth was sealed with a rubber stopper. Vacuumize - fill with nitrogen 3 times to remove all the oxygen in the bottle.
[0106] Inject 40mL of methanol and 100mL of toluene into the bottle with a syringe (the methanol and toluene were passed through nitrogen to remove oxygen in advance), and stirred at room temperature for 30min to fully mix and dissolve the substances in the system. Heated to 120°C, reacted for 10h, and ended the reaction.
[0107] The toluene was spin-dried, and the resulting solid was dissolved in dichloromethane (100 mL), washed with a 5% HCl solution of dilute hydrochloric acid (500 mL) to remove Na 2 CO 3 . Separ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com