Method for extracting compounds with pregnane mother nucleus structure from compositions
A composition and compound technology, applied in the field of analytical chemistry, can solve the problems of high noise, interference, inaccuracy, etc., and achieve the effect of clean baseline, stable sample solution and high number of theoretical plates.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
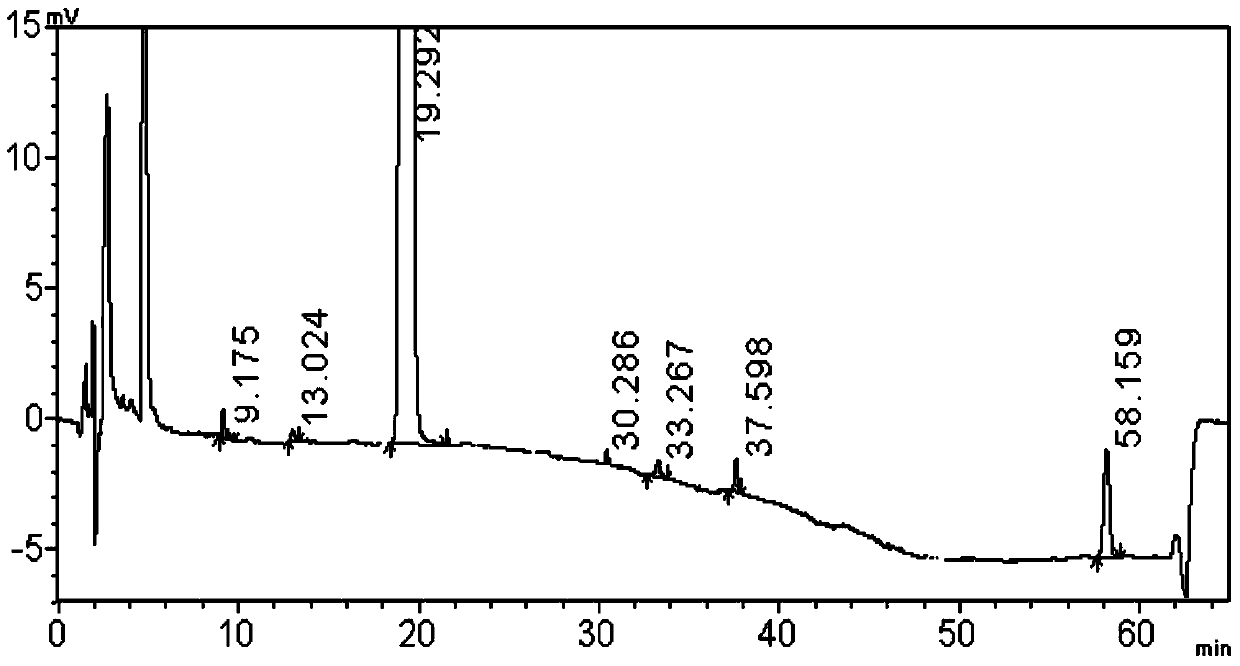
[0080] Embodiment 1 Determination of related substances in halometasone cream
[0081] (1) Chromatographic method
[0082] Chromatographic column: Shimpack VP-ODS (250mm×4.6mm, 5μm)
[0083] UV detector: 254nm
[0084] Column temperature: 25°C
[0085] Flow rate: 1.5ml / min
[0086] Injection volume: 20μl
[0087] System suitability: The resolution of Halometapine peak and adjacent impurity peak should reach 1.5.
[0088] Mobile phase: Phase A is 0.1% glacial acetic acid solution, phase B is acetonitrile, and the gradient elution is performed as follows:
[0089]
[0090] (2) Extraction and testing
[0091] Get about 10 g of the content of halometasone cream (produced by Chongqing Huabang Pharmaceutical Co., Ltd., specification 0.05%) (approximately equivalent to halometasone 5 mg), put it in a stoppered Erlenmeyer flask, add 10 ml of acetonitrile and 0.2 g of sodium chloride, 60 ℃ water bath to dissolve, put it in an ice-water bath for 30 minutes, take it out and sha...
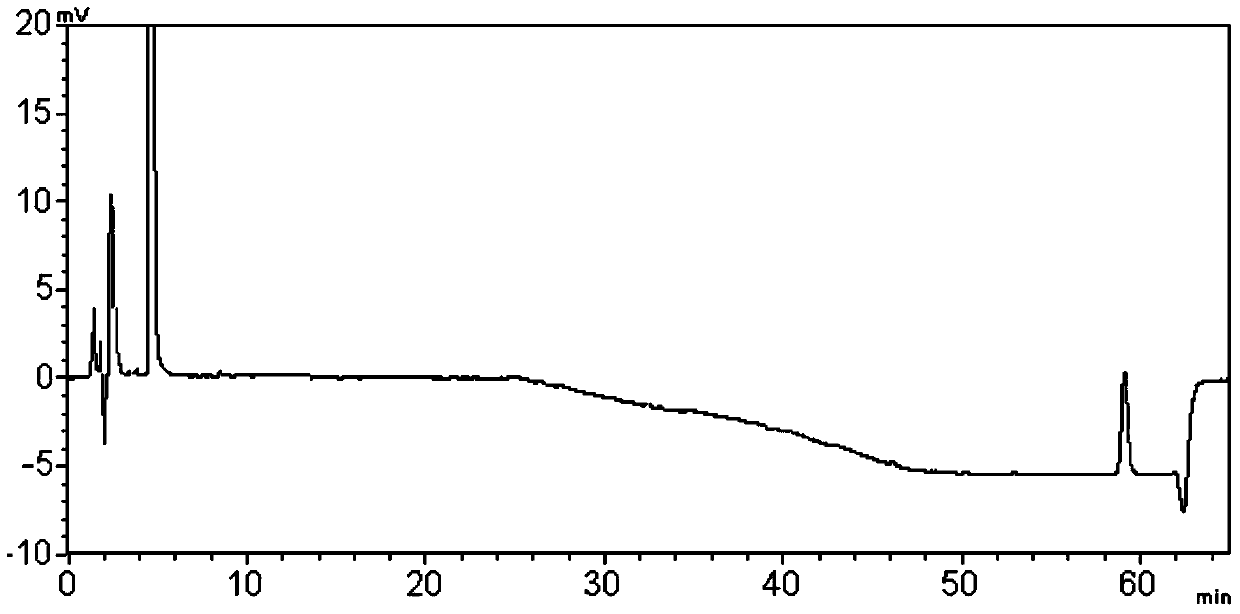
Embodiment 2
[0099] Embodiment 2 Determination of related substances in clobetasone butyrate cream
[0100] (1) Chromatographic method
[0101] Chromatographic column: YMC-Pack ODS-A 150mm×4.6mm, 3.0μm
[0102] Flow rate: 1.5ml / min Column temperature: 40°C
[0103] Detection wavelength: 241nm
[0104] Injection volume: 30μl
[0105] Mobile phase: Phase A of the mobile phase is water, phase B is acetonitrile, and the gradient elution is performed as follows:
[0106]
[0107] (2) Extraction and testing
[0108] To operate in the dark, take an appropriate amount of clobetasone butyrate cream (produced by Sino-US Tianjin SmithKline Pharmaceutical Co., Ltd., specification 0.05%) (approximately equivalent to clobetasone butyrate 10mg), and put it in a stoppered Erlenmeyer bottle Add 16ml of acetonitrile and 0.2g of potassium chloride, dissolve in a water bath at 60°C, place in an ice bath for 60 minutes, take out and shake well, then place in a centrifuge, centrifuge at 4000rpm for 10 m...
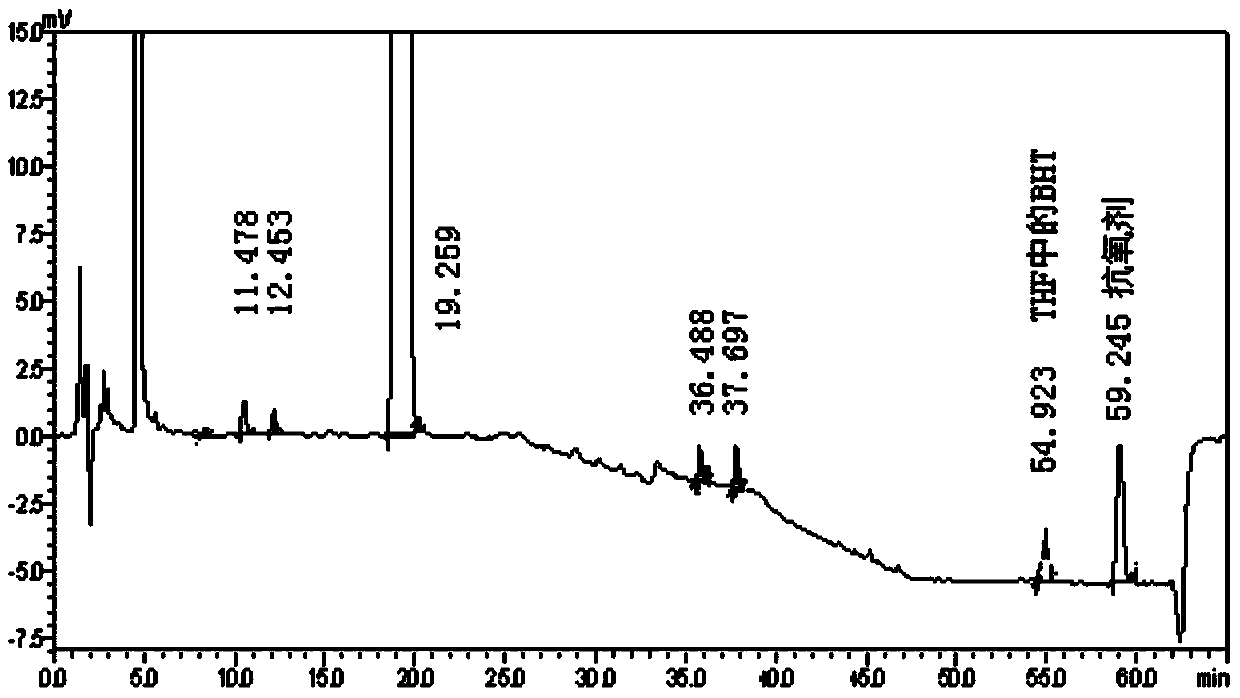
Embodiment 3
[0117] Example 3 Determination of related substances in betamethasone dipropionate cream
[0118] (1) Chromatographic method
[0119] Chromatographic column: Agilent XDB-C18 (250mm×4.6mm, 5μm)
[0120] UV detector: 240nm
[0121] Column temperature: 25°C
[0122] Flow rate: 1.5ml / min
[0123] Injection volume: 20μl
[0124] Mobile phase: 45% acetonitrile
[0125] (2) Extraction and testing
[0126] Take about 10 g of the content of betamethasone dipropionate cream (produced by Chongqing Huabang Pharmaceutical Co., Ltd., specification 0.1%) (approximately equivalent to 10 mg of betamethasone dipropionate), put it in a stoppered Erlenmeyer flask, add 10 ml of acetonitrile and sodium chloride 0.2g, dissolved in a water bath at 60°C, placed in an ice-water bath for 30 minutes, taken out and shaken evenly, allowed to cool to room temperature, filtered the clear solution, and took the subsequent filtrate as the test solution. Precisely measure 1.0ml of the test solution, put ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com