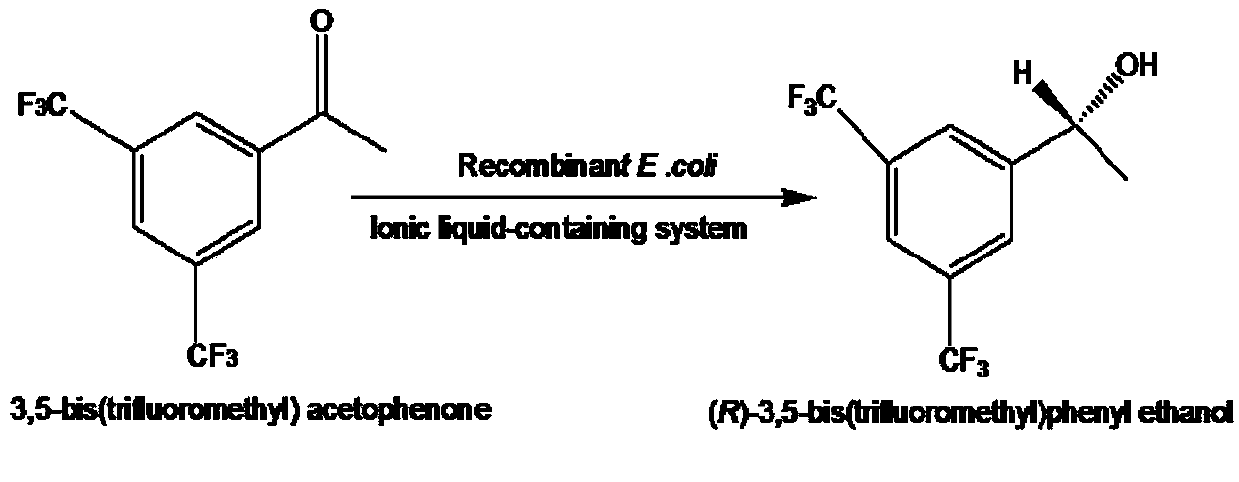
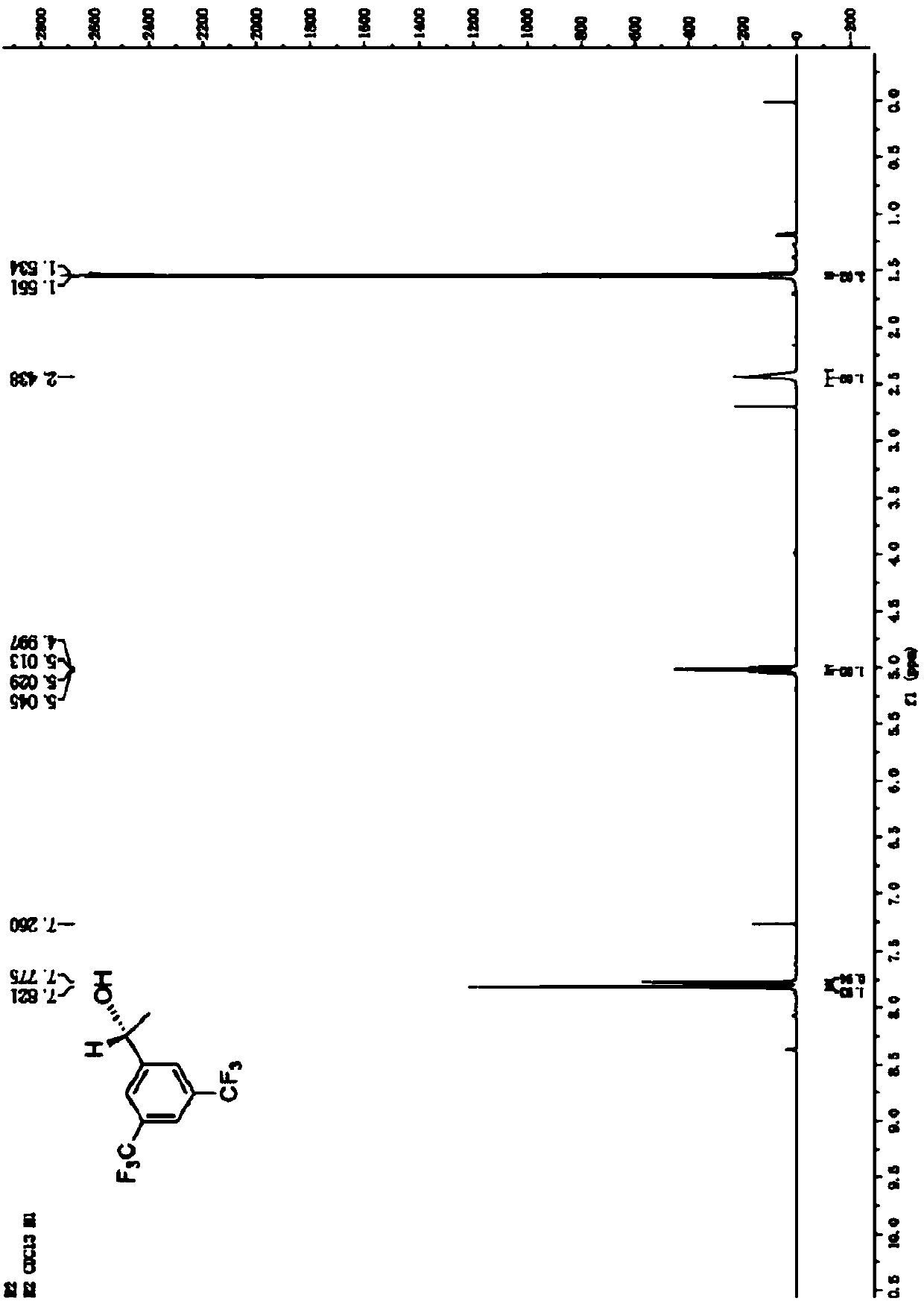
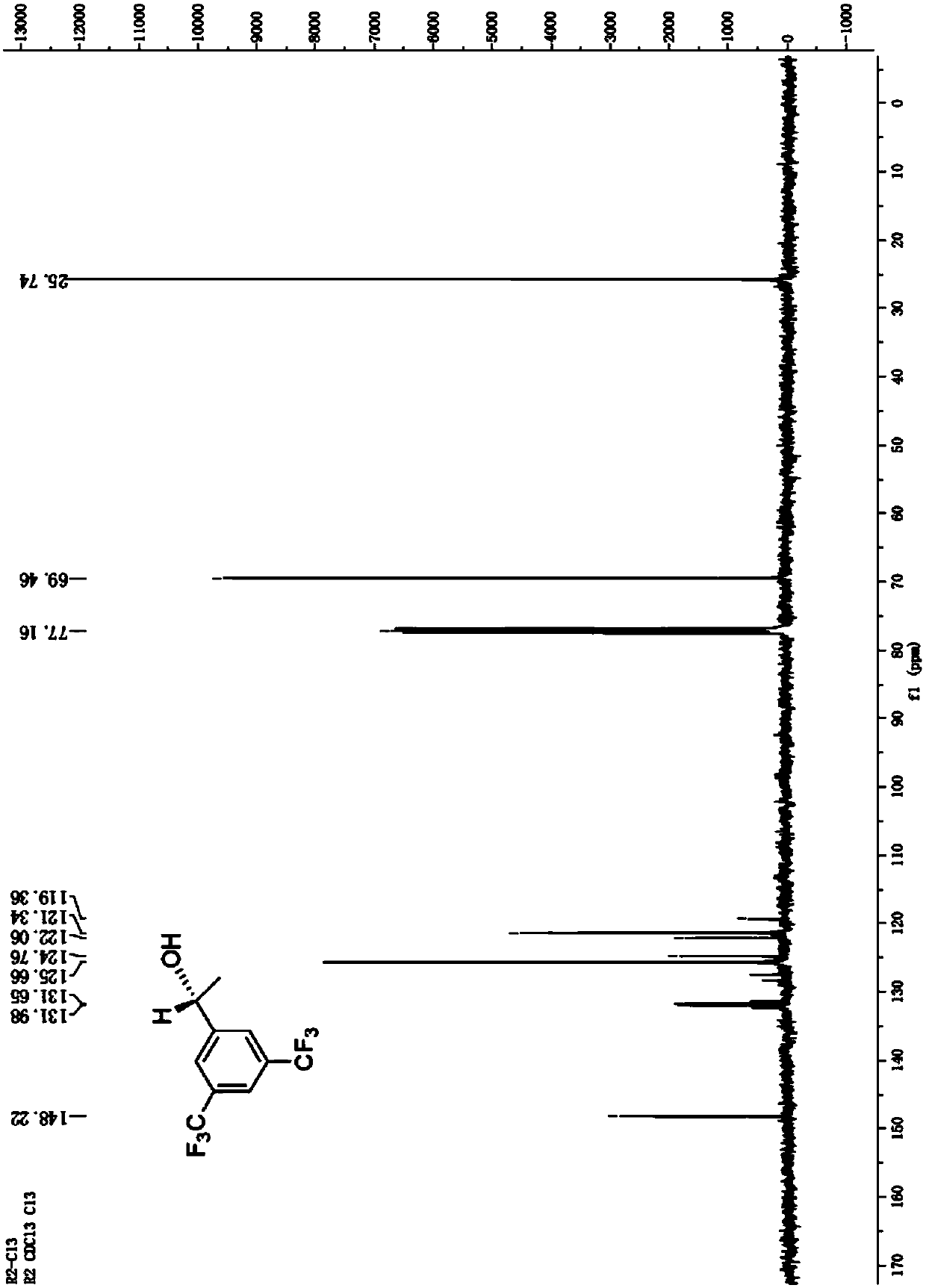
Method for preparing (R)-3,5-bis(trifluoromethyl)phenethyl alcohol in ionic liquid-containing cosolvent medium
A technology of trifluoromethyl phenethyl alcohol and ionic liquid, which is applied in the field of biological reduction to prepare -3,5-bis-trifluoromethyl phenethyl alcohol, can solve problems such as low yield, reduce inhibition effect and increase substrate concentration , the effect of improving the reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: Construction of Leifsonia xyli (Leifsonia xyli) HS0904 short-chain dehydrogenase mutant
[0032] Using the plasmid pET28a(+) containing short-chain dehydrogenase derived from Leifsonia xyli HS0904 as a template, the mutant LXCAR was obtained by error-prone PCR and site-directed mutagenesis. The C at position 461 in the nucleotide sequence is mutated to A, and the G at position 462 is mutated to T; the serine at position 154 in the corresponding amino acid sequence is mutated to tyrosine (the nucleotide sequence is shown in SEQ ID NO.3, encoding The amino acid sequence of the protein is shown in SEQ ID NO.4). The obtained short-chain dehydrogenase mutant gene was ligated with the expression plasmid pET28a(+) and then transformed into Escherichia coli BL21(DE3), thereby constructing the short-chain dehydrogenase mutant containing Leifsonia xyli HS0904 Recombinant Escherichia coli BL21(DE3) / pET28a(+)-LXCAR with body gene.
Embodiment 2
[0033] Embodiment 2: the preparation method of wet thalline used in the embodiment of the present invention
[0034] The recombinant Escherichia coli BL21(DE3) / pET28a(+)-LXCAR obtained from the construction was inoculated in LB liquid medium containing 50 μg / mL kanamycin, cultured with shaking at 37°C and 200 rpm for 12 hours, and then injected with 1% volume concentration of The inoculum was inoculated into fresh LB liquid medium containing 50 μg / mL kanamycin, and cultured at 37°C and 200 rpm to the cell concentration OD 600 After the temperature is about 0.6 to 0.9, add isopropyl-B-D-thiogalactopyranoside (IPTG) at a final concentration of 0.1 mmol / L to the culture medium, and place it at 30°C and 200 rpm for 10 hours to induce culture. After the cultivation, centrifuge at 4°C and 10,000 rpm for 10 minutes to collect the cells, wash the cells with physiological saline twice, and collect the wet cells, which are wet cells of recombinant Escherichia coli.
Embodiment 3
[0036] Respectively, the recombinant engineering bacteria (before mutation) containing the short-chain dehydrogenase gene derived from Leifsonia xyli (Leifsonia xyli) HS0904 and the mutant gene containing the short-chain dehydrogenase derived from Leifsonia xyli (Leifsonia xyli) HS0904 Recombinant engineered bacteria (after mutation) are used as biocatalysts to prepare (R)-3,5-bistrifluoromethylphenethyl alcohol ((R) by catalytic asymmetric reduction of 3,5-bistrifluoromethylacetophenone (BTAP) )-BTPE) performance comparison.
[0037] The method is as follows (the total volume of the reaction system is 5 mL): in a 50 mL Erlenmeyer flask, add 20 g / L (wet weight) of recombinant E. coli wet thallus, 3.92 mL of phosphate buffer (200 mM, pH7.2), 20% (v / v ) of isopropanol and 200 mM of the substrate (BTAP). React at 30° C. and 200 rpm for 4 hours. After the reaction, the reaction solution was extracted with an equal volume of ethyl acetate, and the ethyl acetate phase was used for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com