Acrylated hyaluronic acid hydrogel loaded with nanomedicine and preparation method thereof
A technology of acrylic esterification and hyaluronic acid, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as difficulties in implementation and unacceptability by patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of Acrylate Functionalized Hyaluronic Acid Derivatives:
[0034] A: Completely dissolve 2g hyaluronic acid in 400mL deionized water, add 30g adipic dihydrazide, dissolve completely and adjust the pH to 5-7 with hydrochloric acid, then add 6.0g carbodiimide hydrochloride to maintain the pH at 5 -7 until it stabilizes;
[0035] B: react at room temperature for 8-15 hours, dialyze with deionized water for 60-80 hours, freeze-dry to obtain hyaluronic acid-adipate dihydrazide derivatives;
[0036] C: Dissolve 1.1915g of hydroxyethylpiperazine thiosulfate, 4.3875g of sodium chloride and 1.8612g of ethylenediaminetetraacetic acid in 500mL of deionized water as a buffer for hydroxyethylpiperazine thiosulfate;
[0037] D: Dissolve 1900mg of hyaluronic acid-adipate dihydrazide derivative in 347.7mL of hydroxyethylpiperazine ethylsulfuric acid buffer, and dissolve 1330mg of N-acryloxysuccinate in 10-20mL of dimethyl sulfoxide Imide, adding N-acryloyloxysuccinimide to...
Embodiment 2
[0041] Acrylated hyaluronic acid hydrogel loaded with silibinin nanoparticles and a preparation method thereof, the steps are as follows:
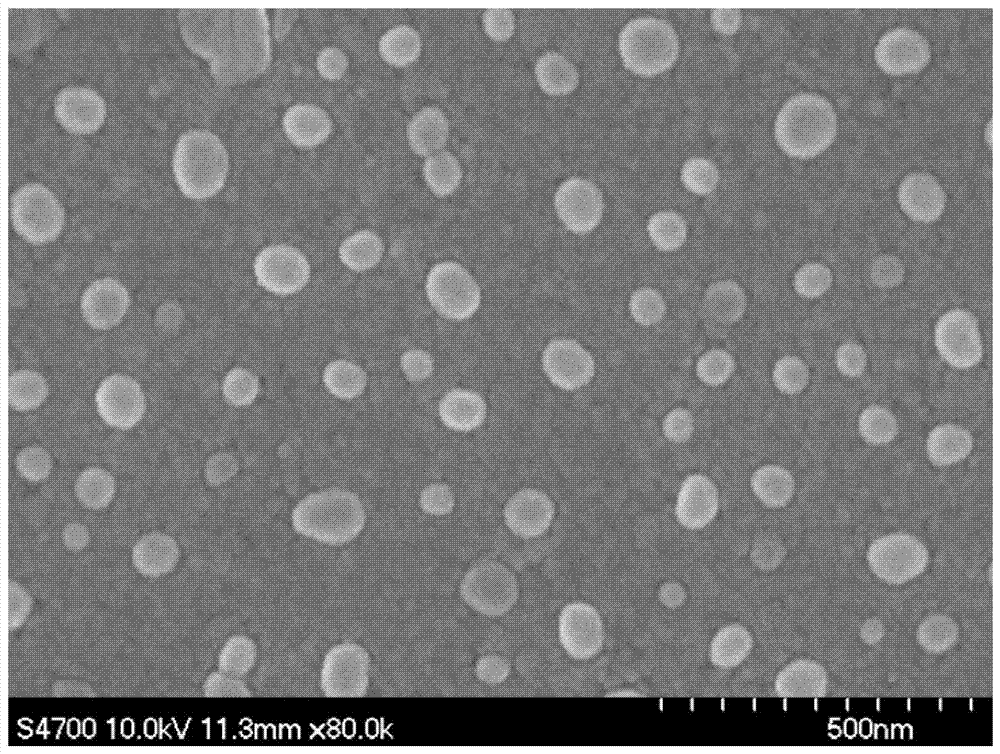
[0042] A: Dissolve 100 mg of acrylate-functionalized hyaluronic acid derivatives in 1.2 mL of 10% sodium bicarbonate buffer solution to obtain a solution of acrylate-functionalized hyaluronic acid derivatives, and compound 121 mg of silybin with an average particle size of 37 nm The powder was dissolved in 400uL sodium bicarbonate buffer to obtain a silibinin aqueous dispersion, and 5.78mg of 1,4-dithiothreitol was added to 400uL sodium bicarbonate buffer;
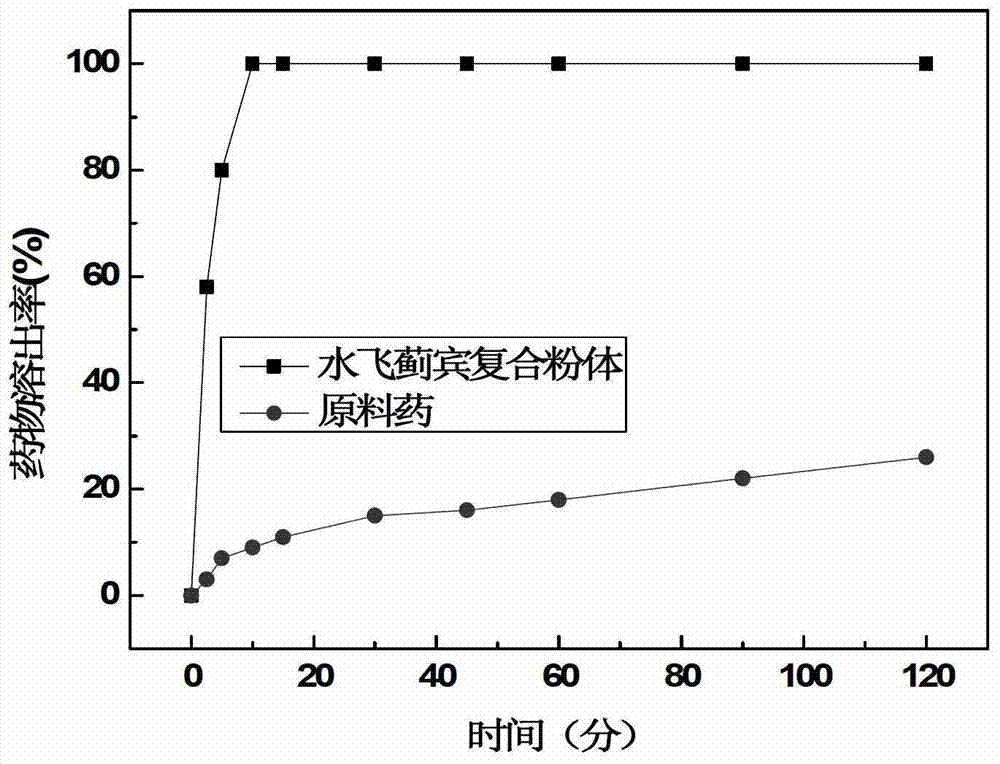
[0043] B: Mix the acrylate-functionalized hyaluronic acid derivative solution and the silibinin water dispersion evenly and add 1,4-dithiothreitol solution. The reaction mixture is reacted at 37°C. The method determines that the gelation time is 6min, and this embodiment gels at 37°C, and the gelation time is short, which is suitable for injection; between the drug nanoparticles and th...
Embodiment 3
[0046] Acrylated hyaluronic acid hydrogel loaded with silibinin nanoparticles and a preparation method thereof, the steps are as follows:
[0047] A: Dissolve 100mg of acrylate-functionalized hyaluronic acid derivatives in 1.2mL of 10% sodium bicarbonate buffer to obtain a solution of acrylate-functionalized hyaluronic acid derivatives, and 121mg of silibinin with an average particle size of 66nm The composite powder was dissolved in 400uL sodium bicarbonate buffer to obtain a silibinin aqueous dispersion, and 5.78mg of 1,4-dithiothreitol was added to 400uL sodium bicarbonate buffer;
[0048] B: Mix the acrylate-functionalized hyaluronic acid derivative solution and the silibinin aqueous dispersion evenly and add 1,4-dithiothreitol, and react the reaction mixture at 37°C by inverting the vial The measured gelation time is 6min. This embodiment gels at 37° C., and the gelation time is relatively short, which is suitable for injection; The weight ratio is 1:5.289;
[0049] C: ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com