Solvate crystal of 9,9-bis(6-hydroxy naphthalene-2-group) fluorene and preparation thereof
A technology of solvate and hydroxynaphthalene, which is applied in the field of solvate crystals of 9,9-bifluorene and its preparation, can solve the problems of deepening color, poor thermal stability, and decreased purity, and achieve good fluidity and thermal stability High performance and high bulk density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
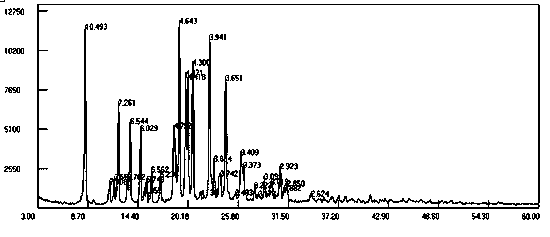
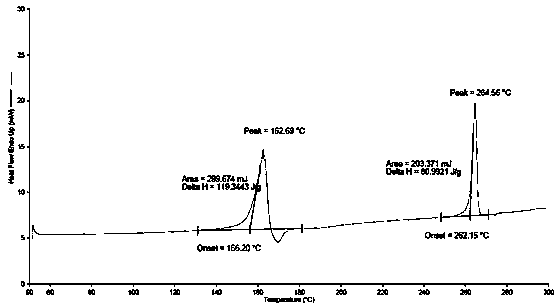
[0022] Add 9-fluorenone (36g, 0.2mol), 2-naphthol (66.24g, 0.46 mol), 3-mercaptopropionic acid (cocatalyst) (2g), toluene 300 mL into the reaction flask, and stir until 9-fluorenone After the ketone and 2-naphthol were dissolved, 39.4 g (0.4 mol) of 98% sulfuric acid was slowly added dropwise. The reaction was stirred at 50-60°C for 4-8 hours. HPLC analysis confirmed that the 9-fluorenone content was below 0.1% and the reaction was stopped. Add 10% sodium hydroxide to neutralize, wash with 3*200 g water at 80°C. Separate the organic layer, evaporate the solvent under reduced pressure, add 200 mL of methanol, and stir to precipitate crystals. Recrystallize with acetonitrile, filter out the crystals, and dry in vacuo at 60°C for 8 hours to obtain 68g of solvate crystal I with a yield of 75.5%, and a purity of 99.5% by high performance liquid chromatography (HPLC). 1 H-NMR) (500Mz, CDCl 3 )δ: 7.03-7.11(m, 4H), 7.38-7.40(m, 2H), 7.50-7.58(m, 14H), 7.83-7.98(d, 2H). The X-ray ...
Embodiment 2
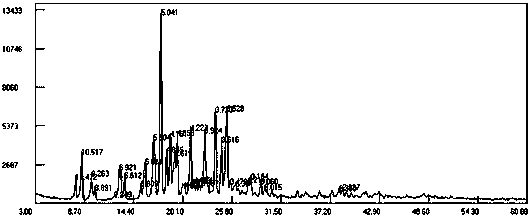
[0024] Add 9-fluorenone (36g, 0.2mol), 2-naphthol (66.24g, 0.46mol), 3-mercaptopropionic acid (co-catalyst) (2g), toluene 300 mL in the reaction flask, stir until 9- After fluorenone and 2-naphthol were dissolved, 39.4 g (0.4 mol) of 98% sulfuric acid was slowly added dropwise. The reaction was stirred at 50-60°C for 4-8h. HPLC analysis confirmed that the 9-fluorenone content was below 0.1% and the reaction was stopped. Add 10% sodium hydroxide to neutralize, wash with 3*200 g water at 80°C. Separate the organic layer, evaporate the solvent under reduced pressure, add 200 mL of methanol, and stir to precipitate crystals. Recrystallize with ethyl acetate, filter out the crystals, and vacuum dry at 60°C for 8 hours to obtain 72g of solvate crystal II with a yield of 80.0% and a purity of 99.6% as analyzed by high performance liquid chromatography (HPLC). H NMR spectrum test ( 1 H-NMR) (500Mz, CDCl 3 ) test, its δ values are 7.01-7.10(m, 4H), 7.35-7.40(m, 2H), 7.48-7.58(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com