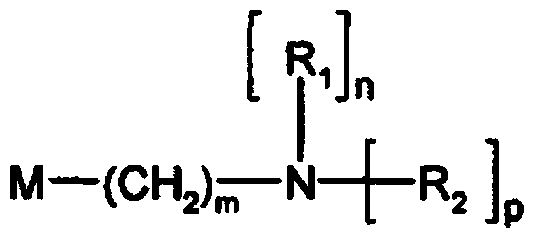
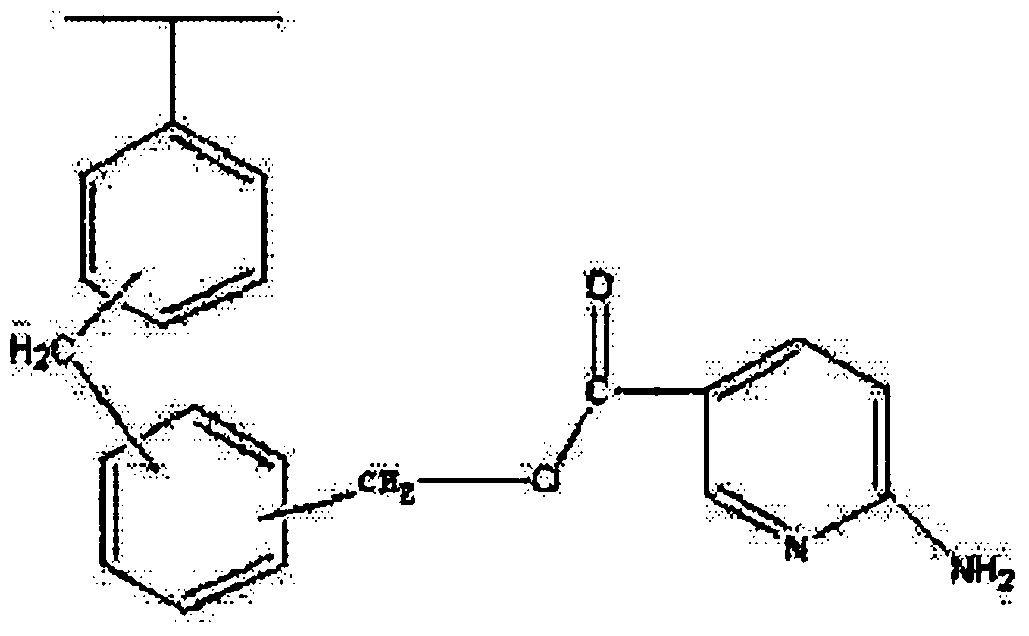
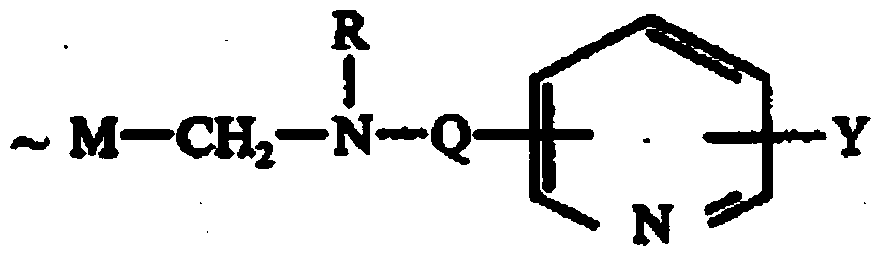Amino nitrogen heterocyclic ring resin and preparation method thereof
A nitrogen heterocycle and nitrogen heterocycle compound technology, applied in the field of organic compound preparation, can solve problems such as low adsorption capacity, poor adsorption selectivity, and reduced resin adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Add 500ml of 8% sodium chloride solution configured with tap water into a 1000ml reactor, then add 1g of hydroxyethyl cellulose and 2.5g of gelatin, stir and heat up to 45°C to dissolve evenly, then stop stirring for subsequent use; Weigh 8.8g of 80% divinylbenzene and 91.2g of styrene in the dried beaker, add 80g of toluene and 0.8g of dibenzoyl peroxide, stir evenly, add them into a 1000ml reaction kettle, let stand for 10min, start stirring and Adjust the stirring speed to disperse the oil phase into spherical particles of 0.2-0.7mm, raise the temperature to 80°C and keep it for 3h, and after the balls solidify, raise the temperature to 90°C to continue the reaction for 5h. After the reaction, the solidified balls were filtered out, and the balls were washed with methylal to clean the toluene, and then washed with water until there was no methylal, to obtain 196 g of the resin matrix.
[0077] Put the cured balls prepared above in an oven at 105°C for 3 hours, measur...
Embodiment 2
[0086] Use commercially available 122 resin and dry it with a moisture content of 0.8%. Weigh 80g of dried resin, add it to a 1000ml three-necked glass reactor, add 400ml of ethylene glycol, start stirring, swell for 1h, then add 300ml of ethylenediamine, heat up to 150°C for 15h, filter out the resin and wash with water until neutral , Obtain 267g primary amine phenolic resin.
[0087] Weigh 100g of the above-mentioned primary amine phenolic resin, add it into a 500ml three-necked glass reactor, use 4-chloropiperidine hydrochloride, and add 60g of 35% potassium hydroxide solution into the reactor, stir and heat up to 40°C; Feed a total of 60 g of an aqueous solution containing 32 g of 4-chloropiperidine hydrochloride into a 500 ml reactor, and after 5 hours, the feeding is completed, and then the temperature is raised to 60 ° C, and the reaction is completed for 18 hours. After the end, the resin is filtered out, washed with ethanol, and washed with water to obtain 108g of p...
Embodiment 3
[0093] According to the preparation method of Example 1, the primary amine resin was prepared, but in the preparation process, the raw materials were adjusted as follows: the addition of 80% divinylbenzene was 7.5g, the addition of styrene was 92.5g, the addition of toluene was 100g, other The raw materials are unchanged. The exchange capacity of the obtained primary amine resin was 7 mmol / g, and the water content was 57.23%.
[0094] Add 100 g of the above-prepared primary amine resin into a 500 ml reaction kettle, add 90 g of 50% sodium carbonate, and start stirring; prepare 65.7 g of 2-chloro-1,3-dimethyl imidazoline chloride into a water-saturated The solution was fed into the reaction kettle within 6 hours, and then heated to 30°C for 35 hours of reaction, washed with ethanol, and washed with water to obtain 149 g of imidazole nitrogen-containing heterocyclic resin.
[0095] Take 100g of the above resin, dry it in an oven, put it into a 500ml reaction kettle, add 74g of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com