Method for producing hydrogen and oxygen through solar photocatalysis of water based on metal oxide photocatalyst
A photocatalyst and oxide technology, which is applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc. Many problems, such as low catalyst stability, can achieve the effect of simple loading method, simple preparation process and wide absorption spectrum range.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Active component rutile phase TiO 2 Preparation of:
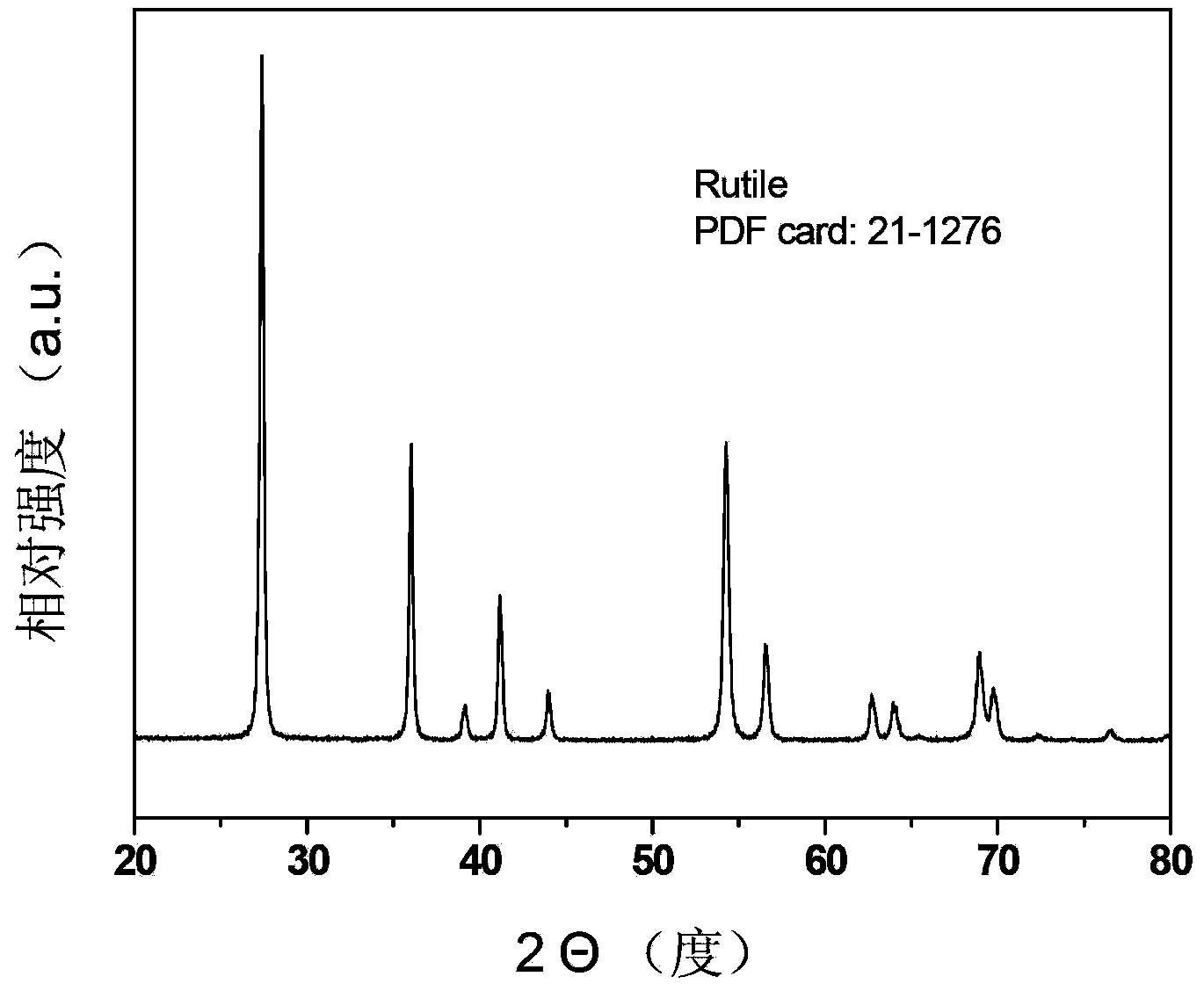
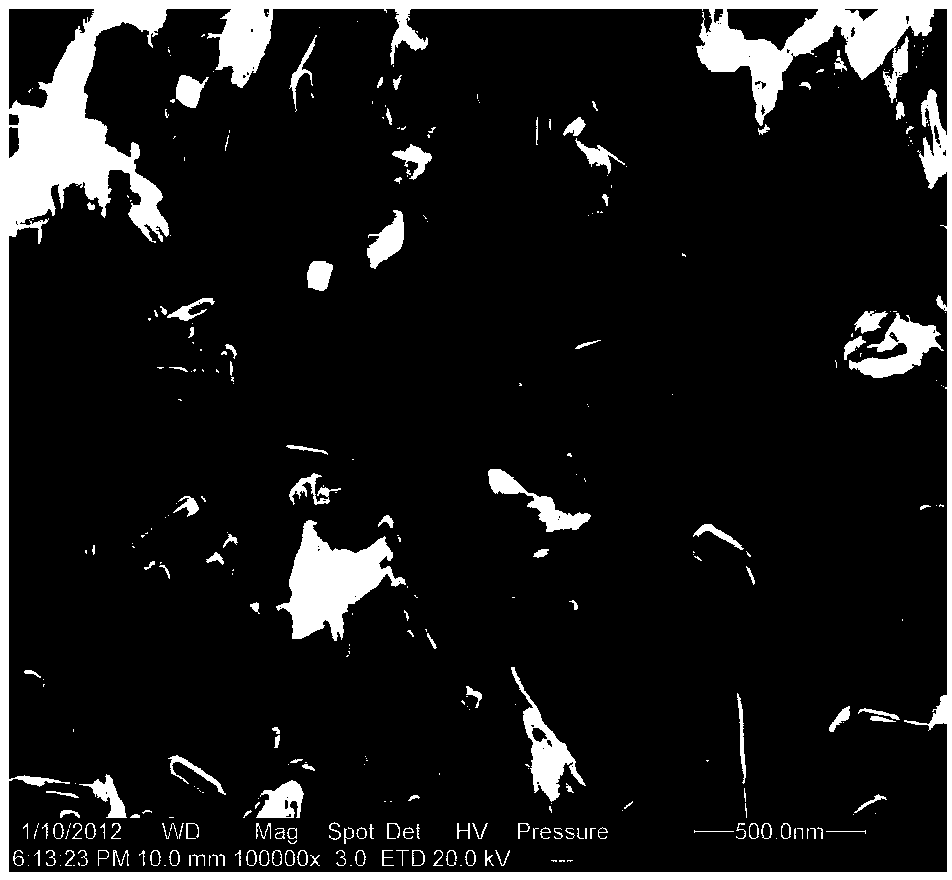
[0043] Mix 6ml of HCl with a concentration of 37wt% and 15ml of titanium isopropoxide for hydrothermal synthesis. The hydrothermal synthesis condition is 180°C, and the reaction is 36h. figure 1 Its XRD diffraction pattern, figure 2 Its scanning electron microscope photograph.
Embodiment 2
[0045] Evaluation of preparation of single noble metal co-catalyst by in situ photodeposition and its photocatalytic reaction
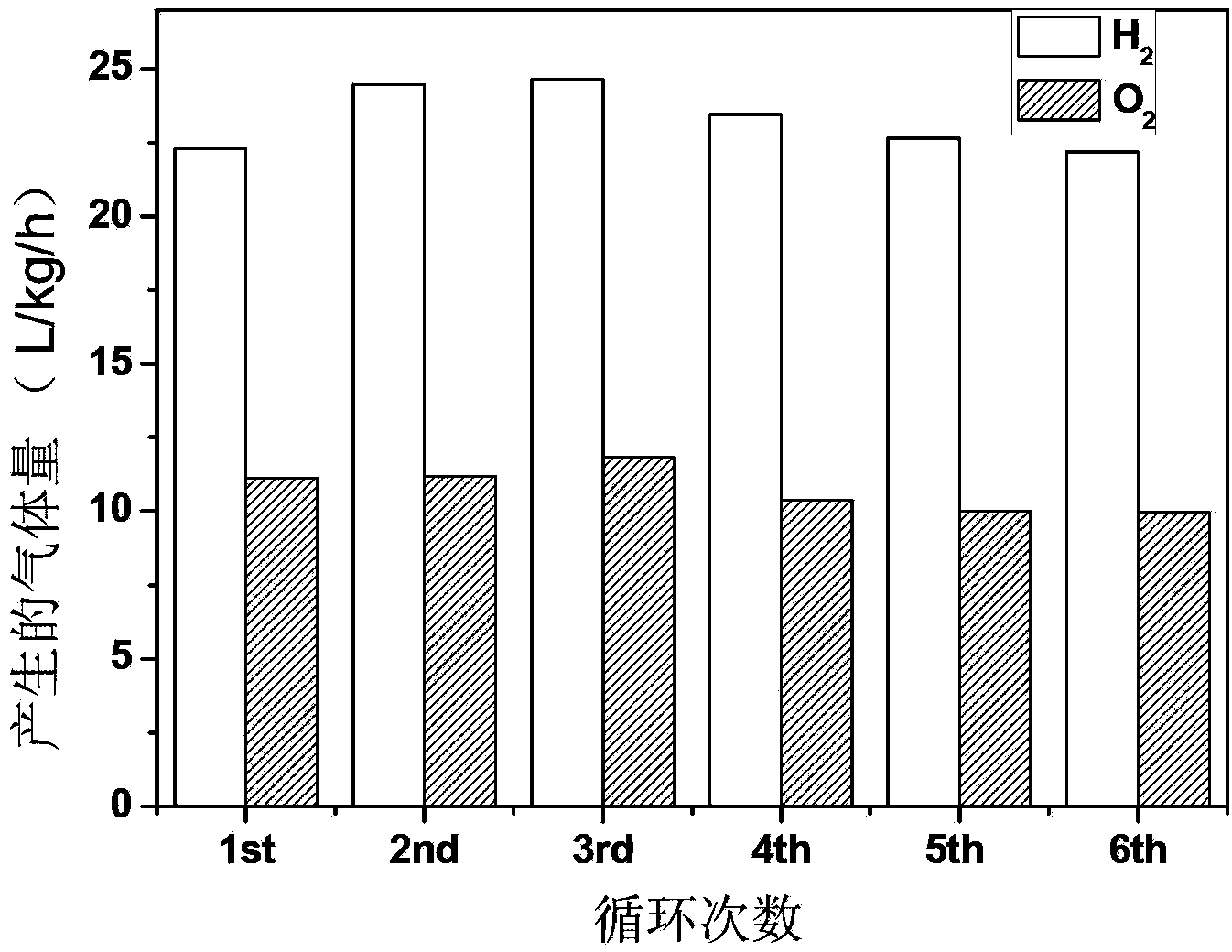
[0046] Add 0.050 g of catalyst and a metal precursor with a single-component metal content of 0.2 wt % in 150 ml of deionized water. The amount added is: 0.27ml H 2 PtCl 6 solution (0.37mg / ml), 0.14ml AgNO 3 solution (0.74mg / ml), 0.44ml HAuCl 4 solution (0.23mg / ml), 0.52ml RhCl 3 solution (0.19mg / ml), 0.17ml RuCl 3 One of the solutions (0.59mg / ml). After the air in the system was evacuated, a 300W xenon lamp was used as the light source for photocatalytic reaction. Among them, the co-catalyst is deposited on the catalyst Cat.0 within 10 minutes at the initial stage of the reaction, and the catalysts Cat1, Cat2, Cat3, Cat4 and Cat5 are obtained, and the corresponding co-catalyst components are: Cat1, Pt; Cat2, Ag; Cat3 , Au; Cat4, Rh; Cat5, Ru. The gas phase products obtained by the reaction were detected online by gas chromatography, and the r...
Embodiment 3
[0048] Preparation of two-component cocatalyst and evaluation of its photocatalytic reaction
[0049] In 150ml deionized water, add 0.050g prepared Cat0, 0.27ml H 2 PtCl 6 solution (0.37mg / ml) and a metal precursor with a second component content of 0.1wt%. The amount added is: 0.10mlKMnO 4 solution (0.50mg / ml), 0.085ml RuCl 3 solution (0.588mg / ml), 0.366ml K 3 IrCl 6 solution (0.273mg / ml) and 0.10ml Cr(NO 3 ) 2 One of the solutions (0.50mg / ml). After the air in the system was evacuated, a 300W xenon lamp was used as the light source for photocatalytic reaction. Among them, the co-catalyst is deposited on the catalyst Cat0 in the initial few minutes of the reaction to obtain the catalysts Cat1-1, Cat1-2, Cat1-3, and 1-4. The corresponding cocatalyst components are: Cat1, Pt; Cat1-1, Pt, Mn 3 o 4 ;Cat1-2,Pt,RuO 2 ;Cat1-3,Pt,IrO 2 ;Cat1-4, Pt, Cr 2 o 3 . Cat1-5 uses IrO 2 Prepared by colloidal adsorption, the specific process is to add Cat0 to the prepared IrO ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com