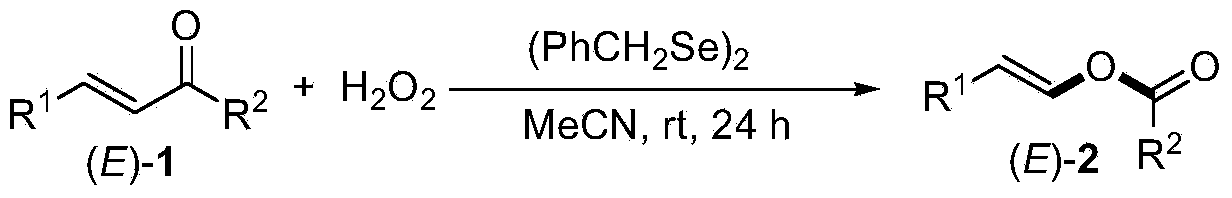Method for synthesizing alkene ester
A technology of enesters and reactants, which is applied in the field of chemical synthesis, can solve problems such as difficulty in large-scale industrial production, narrow application range, and low atom economy, and achieve high yield, wide application range, and high atom economy. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017]
[0018] 1 mmol α, β-unsaturated ketone, 4 mmol hydrogen peroxide (30 w / w%), 0.05 mmol dibenzyl diselenide were stirred in 2 ml acetonitrile at room temperature (25° C.). After 24 hours, the reaction was completed, evaporated to dryness with a rotary evaporator, and the residue was separated by preparative wave chromatography to obtain the enester.
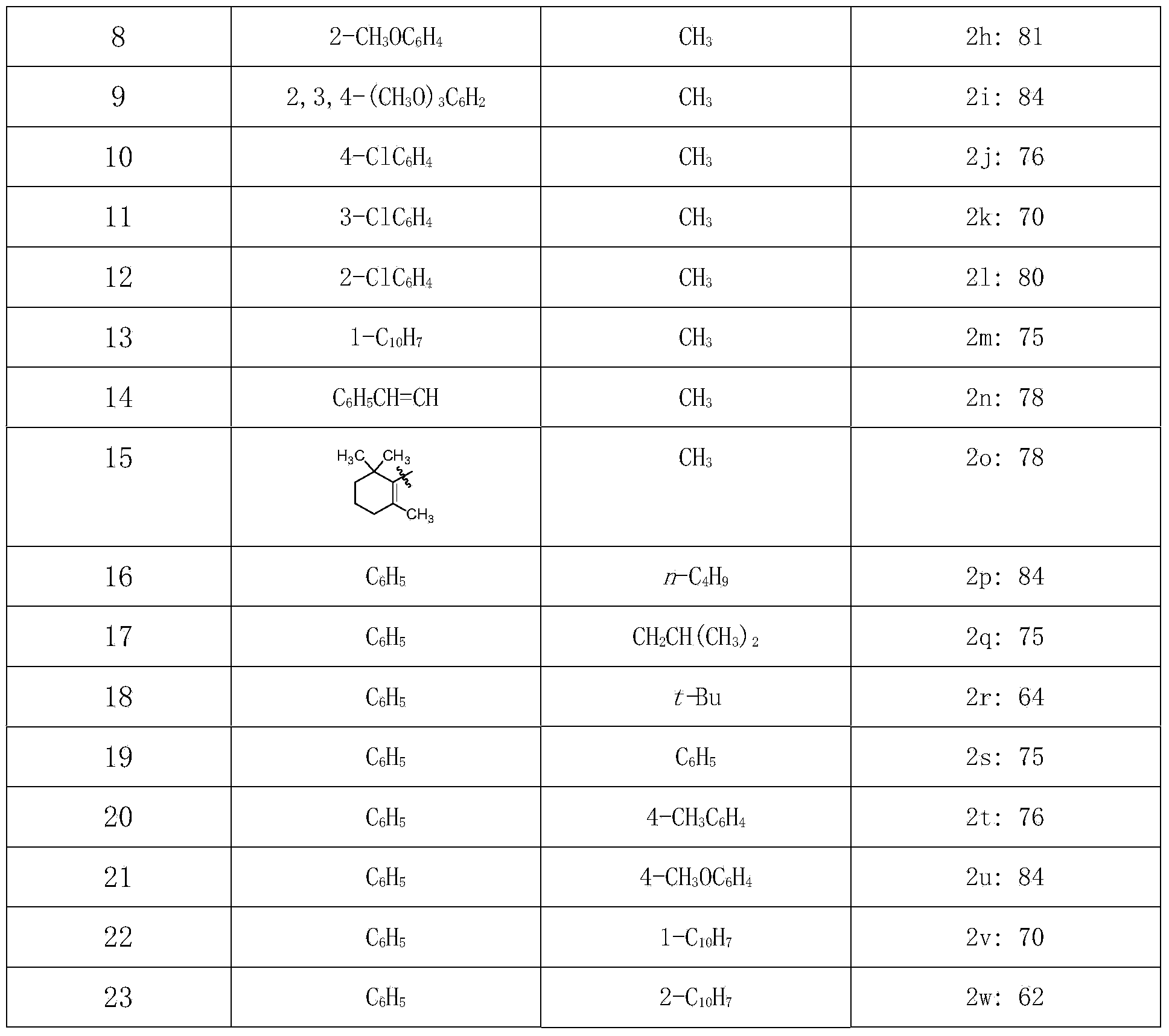
[0019] Check the application range of the reaction substrate, as shown in Table 1, the experimental results of the inspection of the substrate application range.
[0020] Table 1
[0021]
[0022] Note: Yields are isolated yields. From the above results, it can be seen that this reaction has a wide range of applications and is suitable for the synthesis of various enesters.
Embodiment 2
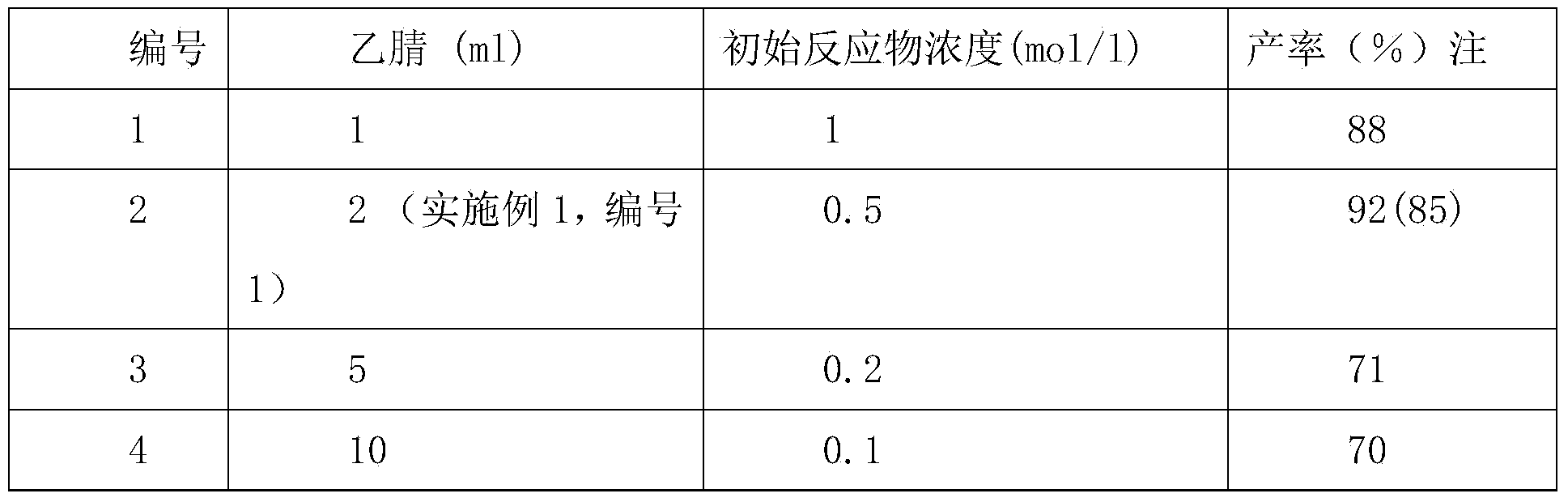
[0024] Other conditions are the same as in Example 1, No. 1. Different volumes of acetonitrile solvents were used, as shown in Table 2 for the experimental results of different volumes of acetonitrile solvents.
[0025] Table 2
[0026]
[0027] Note: The GC yields are outside the brackets, and the separation yields are inside the brackets. As can be seen from the above, the initial reactant concentration is between 0.1-1mol / l, and wherein the effect of the production rate with the initial reactant concentration in No. 1 being 0.5mol / l is the best in Example 1.
Embodiment 3
[0029] Other conditions are the same as embodiment 1, No. 1. Test the reaction using different catalysts, such as Table 3 is the experimental results of the test of different catalysts.
[0030] table 3
[0031] Numbering
[0032] 19
[0033] Note: The GC yields are outside the brackets, and the separation yields are inside the brackets. From the above results, it can be seen that diselenide and organic selenous acid have the best effect, and wherein the yield of dibenzyl diselenide in No. 1 in Example 1 is the best effect.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com