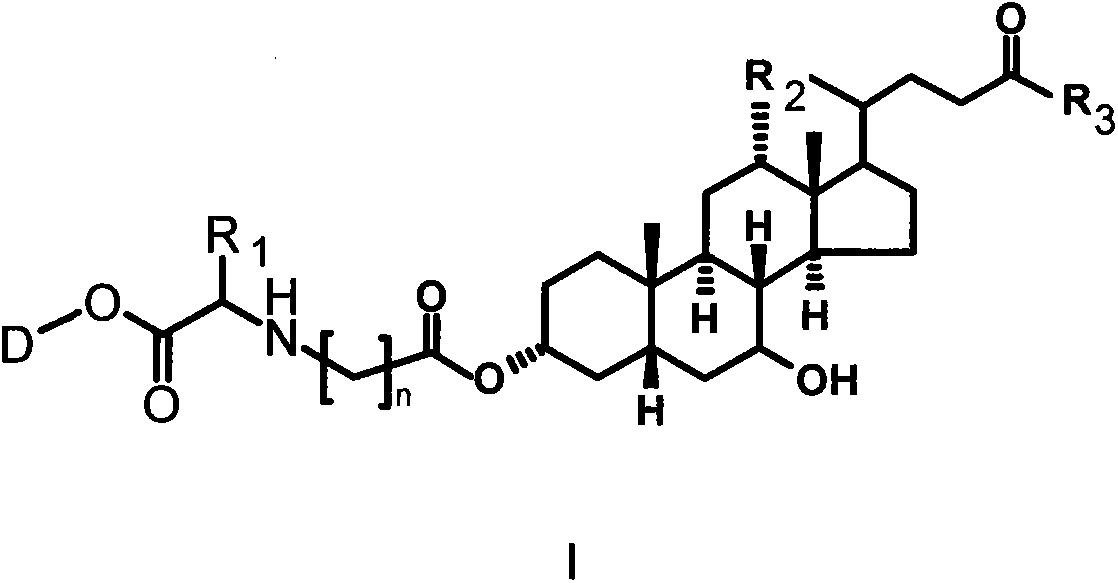
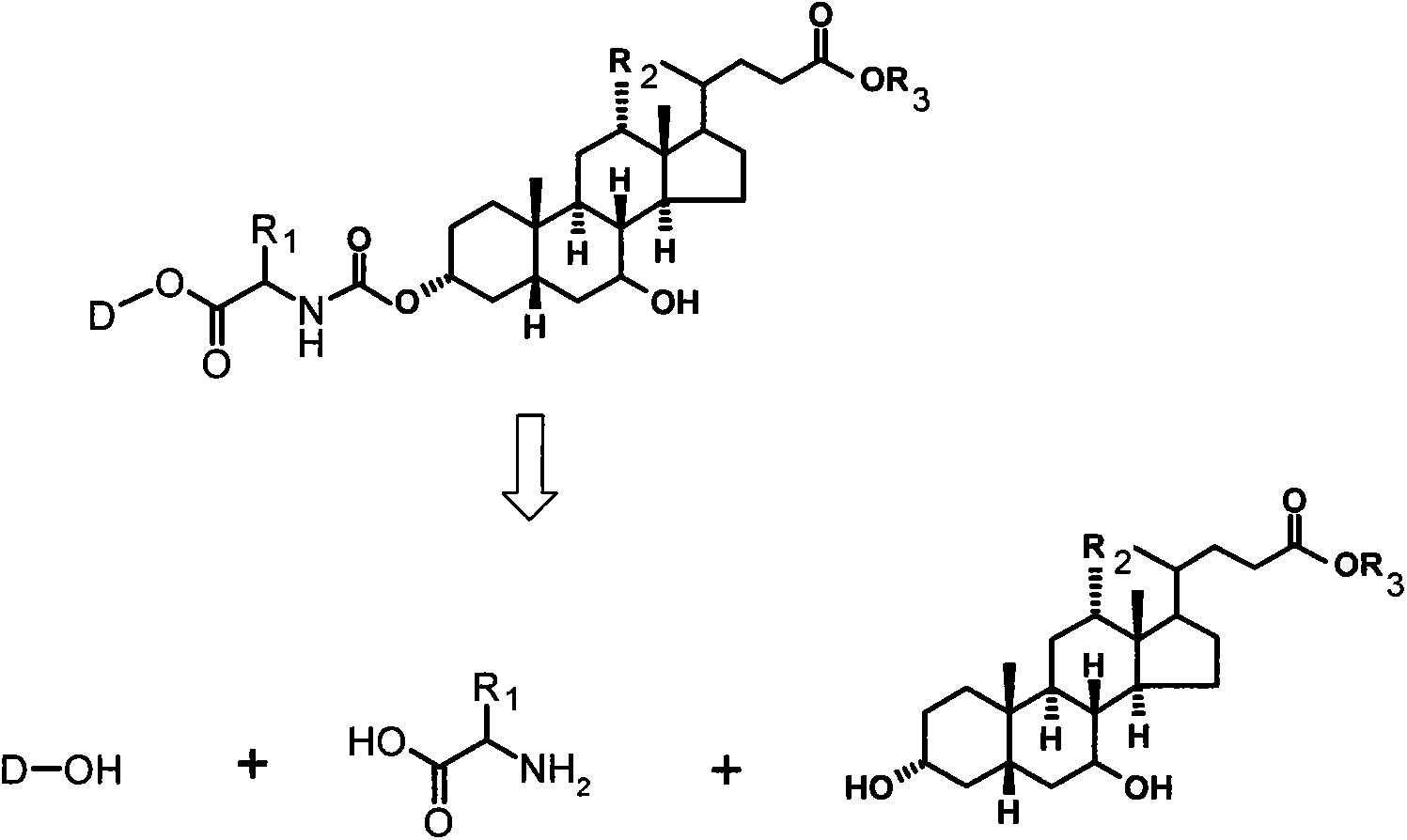
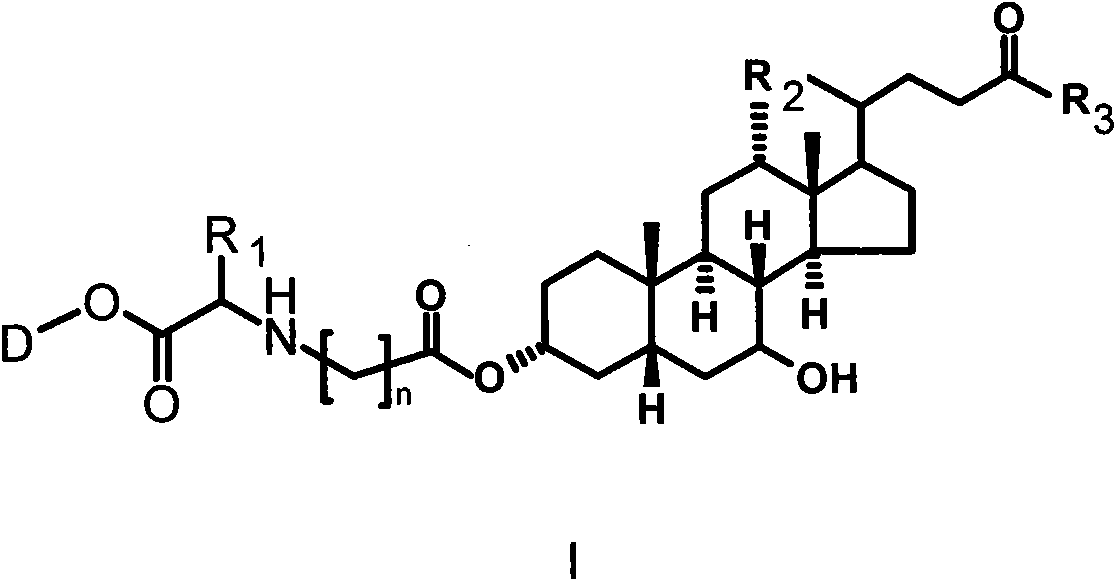
Bile acid-drug conjugate with amino acid as connexon, and medical application thereof
A technology of bile acid and conjugates, which is applied in the field of bile acid-drug conjugates with amino acids as linkers and their medical applications, which can solve the problems of difficult cross-linking, great influence on physical and chemical properties and biological properties, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1 cholic acid-L-alanine-ribavirin conjugate (I 1 ) preparation
[0043]
[0044] 1.1 Synthesis of benzyloxycholate (II 1 )Synthesis
[0045] Dissolve 41g of cholic acid in 200ml of dimethylformamide, then add 20ml of dicyclohexylamine, stir at room temperature, and add 12ml of benzyl bromide dropwise. The reaction was stirred overnight, and the solvent was evaporated under reduced pressure. Add 800ml of ethyl acetate to the residue, stir, and filter; the filtrate is washed with saturated NaHCO 3 Washed with aqueous solution (2X300ml), the organic layer was separated and dried overnight with anhydrous sodium sulfate. The desiccant was filtered off, the filtrate was evaporated to dryness under reduced pressure, separated by silica gel column chromatography, and eluted with a mixed solvent of petroleum ether: ethyl acetate: methanol (10:10:1), the desired components were collected, and evaporated to dryness under reduced pressure , get II 1 36.5 g of wh...
Embodiment 2
[0054] Embodiment 2 cholic acid-L-valine-ribavirin conjugate (I 2 ) preparation
[0055]
[0056] With reference to the method of Example 1.3, replace L-alanine hydrochloride and III with L-valine hydrochloride 1 Reaction, the reaction product is separated and purified to obtain 3-O-((S)-1-carboxy-2-methylpropylaminocarbonyl)-benzyloxycholate IV 2 , yield 32%.
[0057] With reference to the method of embodiment 1.4, use IV 2 instead of IV 1 React with ribavirin, and the reaction product obtains benzyl cholate-L-valine-ribavirin conjugate (V 2 ), yield 58%.
[0058] Referring to the method of Example 1.5, V 2 Catalytic hydrogenation, the reaction product is separated and purified to obtain the target compound I 2 , yield 91%. Elemental analysis (C 38 h 59 N 5 o 12 ) Theoretical value (%): C58.67, H7.64, N9.00; Experimental value (%): C58.92, H7.69 N9.17.
Embodiment 3
[0059] Embodiment 3 cholic acid-L-leucine-ribavirin conjugate (I 3 ) preparation
[0060]
[0061] With reference to the method of Example 1.3, replace L-alanine hydrochloride and III with L-leucine hydrochloride 1 Reaction, the reaction product is separated and purified to obtain 3-O-((S)-1-carboxy-3-methylbutylaminocarbonyl)-benzyloxycholate IV 3 , yield 39%.
[0062] With reference to the method of embodiment 1.4, use IV 3 instead of IV 1 React with ribavirin, and the reaction product is separated and purified to obtain benzyl cholate-L-leucine-ribavirin conjugate (V 3 ), yield 36%.
[0063] Referring to the method of Example 1.5, V 3 Catalytic hydrogenation, the reaction product is separated and purified to obtain the target compound I 3 , yield 93%. Elemental analysis (C 39 h 61 N 5 o 12 ) Theoretical value (%): C59.15, H7.76, N8.84; Experimental value (%): C58.95, H7.90 N9.11.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com