A kind of preparation method of 7a-double condensed pyrrolidine-acetonitrile
A technology of pyrrolidine and acetonitrile, which is applied in the field of synthesizing high-purity 7A-double-condensed pyrrolidine-acetonitrile, can solve the problems of poor industrialization prospects, reduced safety, difficult purification, etc., and achieves convenient purification and separation, improved stability, Effects of avoiding freezing conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
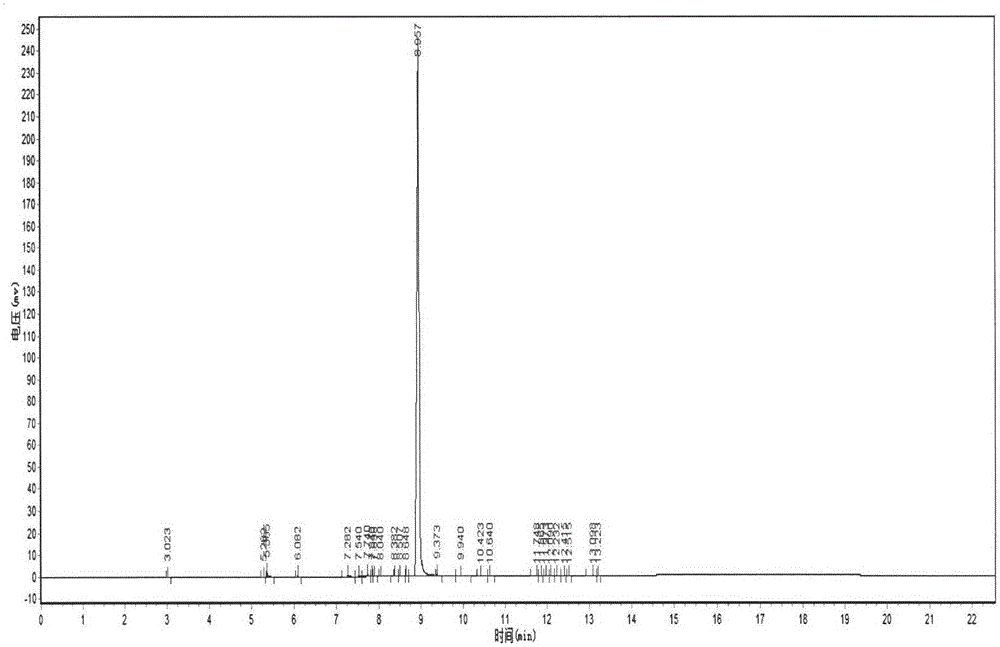
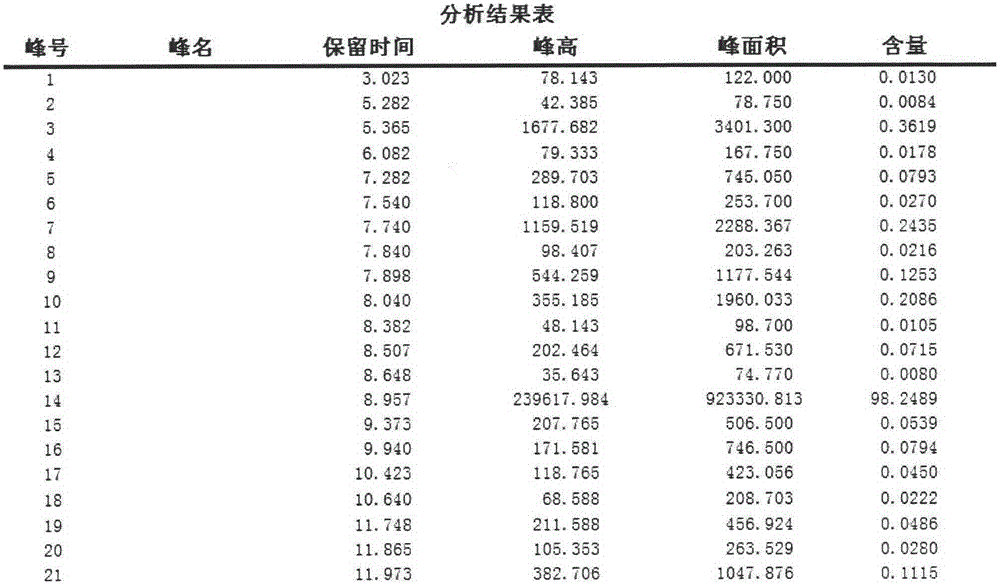
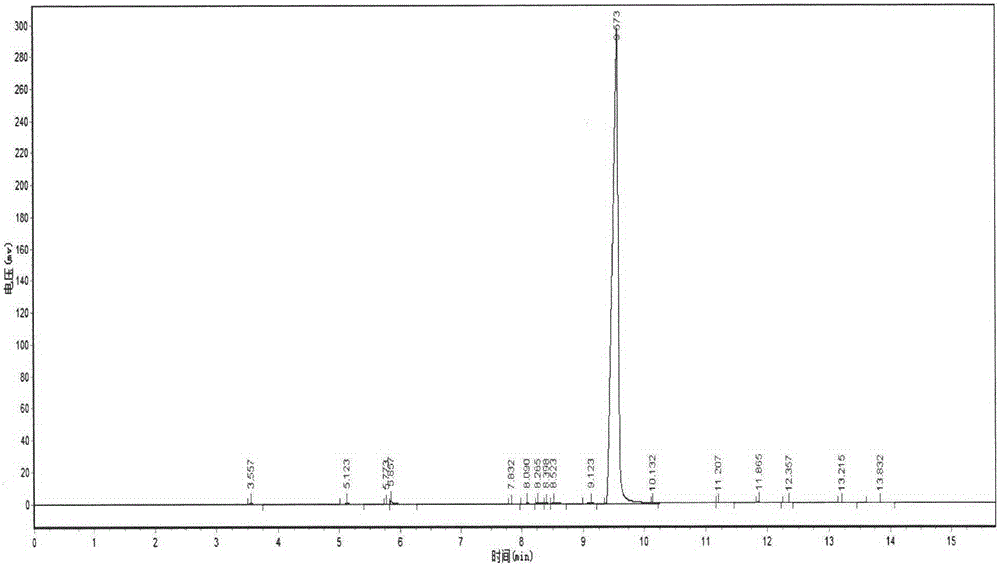
Image
Examples
Embodiment 1
[0034] first step:
[0035] Pump 400kg of γ-butyrolactone and 320kg of anhydrous methanol into a 2000L reactor, stir, turn on and cool to below 25°C, then pump in 420kg of 30% sodium methoxide solution (28%-30%), and control the temperature below 35°C. After the addition, the water bath was heated to 65°C and kept for 4h. Begin to concentrate and distill to recover the solvent under reduced pressure (most of the solvents are recovered at an internal temperature lower than 45-55°C, keeping at 60°C for a long time may affect the yield). The solvent was concentrated as much as possible (vacuum -0.092 or more, until no obvious solvent dripping out). Add 300Kg of ethanol to dissolve, press filter after stirring to obtain about 235kg of crude product, the GC purity is about 80%.
[0036] Step two:
[0037] Add 400kg of formic acid and 110kg of ammonium formate to a 5000L reactor, cool to 0-5°C, and add 450kg of CMP-1. After the addition, the reaction was heated to reflux for 48 ...
Embodiment 2
[0041] first step:
[0042]Inject 400kg of γ-butyrolactone and 320kg of anhydrous methanol into the 2000L reactor, stir, turn on and cool to below 25°C, then pump in 420kg of 30% sodium methoxide solution (28%-30%), and control the temperature below 35°C. After the addition, the water bath was heated to 65°C and kept for 4h. Begin to concentrate and distill to recover the solvent under reduced pressure (most of the solvents are recovered at an internal temperature lower than 45-55°C, keeping at 60°C for a long time may affect the yield). The solvent was concentrated as much as possible (vacuum -0.092 or more, until no obvious solvent dripping out). Add 500Kg of methanol to dissolve, stir and freeze to precipitate crystals to obtain about 250kg (245-275kg) of crude product, with a GC purity of about 94%.
[0043] Step two:
[0044] Add 400kg of formic acid and 110kg of ammonium formate to a 5000L reactor, cool to 0-5°C, and add 450kg of CMP-1. After the addition, the reacti...
Embodiment 3
[0048] first step:
[0049] Pump 400kg of γ-butyrolactone and 320kg of anhydrous methanol into the 2000L reactor, stir, turn on and cool to below 25°C, then pump in 420kg of 30% sodium methoxide solution, and control the temperature below 35°C. After the addition, the water bath was heated to 65°C and kept for 4h. Begin to concentrate and distill to recover the solvent under reduced pressure (most of the solvents are recovered at an internal temperature lower than 45-55°C, keeping at 60°C for a long time may affect the yield). The solvent was concentrated as much as possible (vacuum -0.092 or more, until no obvious solvent dripping out). Add 500 Kg of methanol / isopropanol mixed solvent to dissolve, stir and freeze to precipitate crystals to obtain about 265 kg of crude product with a GC purity of about 96%.
[0050] Step two:
[0051] Add 400kg of formic acid and 110kg of ammonium formate to a 5000L reactor, cool to 0-5°C, and add 450kg of CMP-1. After the addition, the re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com