Preparation method of high-specific surface nanometer magnesium ferrite catalyst material capable of being used in solid propellant
A solid propellant, high specific surface technology, applied in nanotechnology for materials and surface science, chemical instruments and methods, physical/chemical process catalysts, etc., can solve problems such as difficult promotion and application, cumbersome operation, and high cost , to achieve the effects of easy large-scale production, simple process and good crystallinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
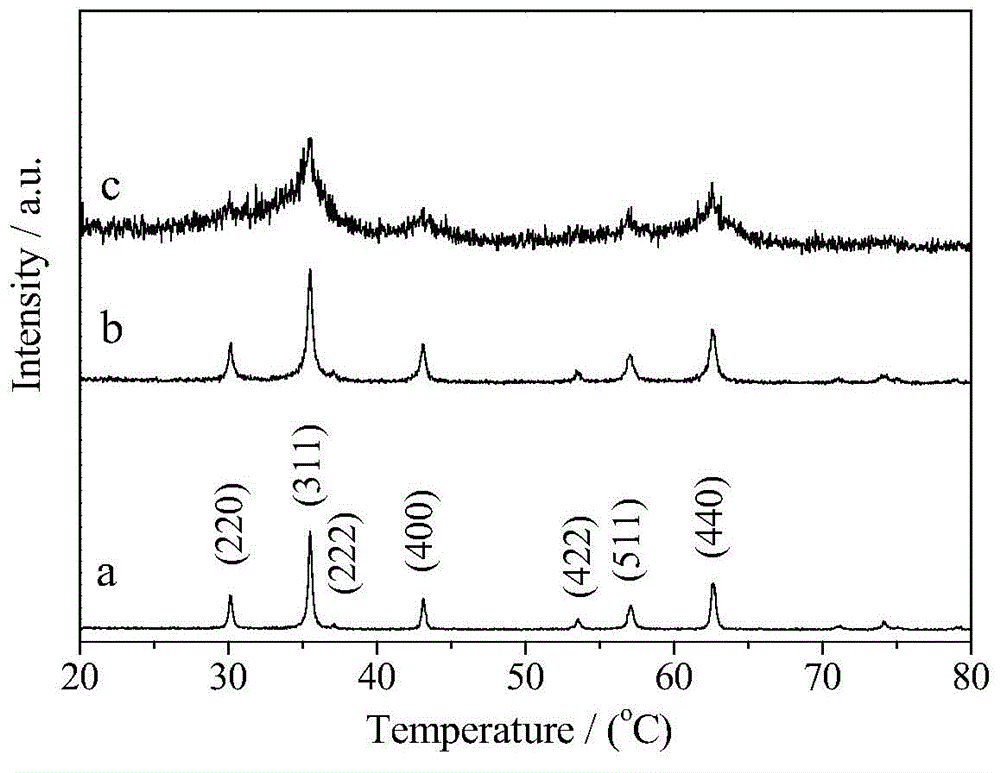
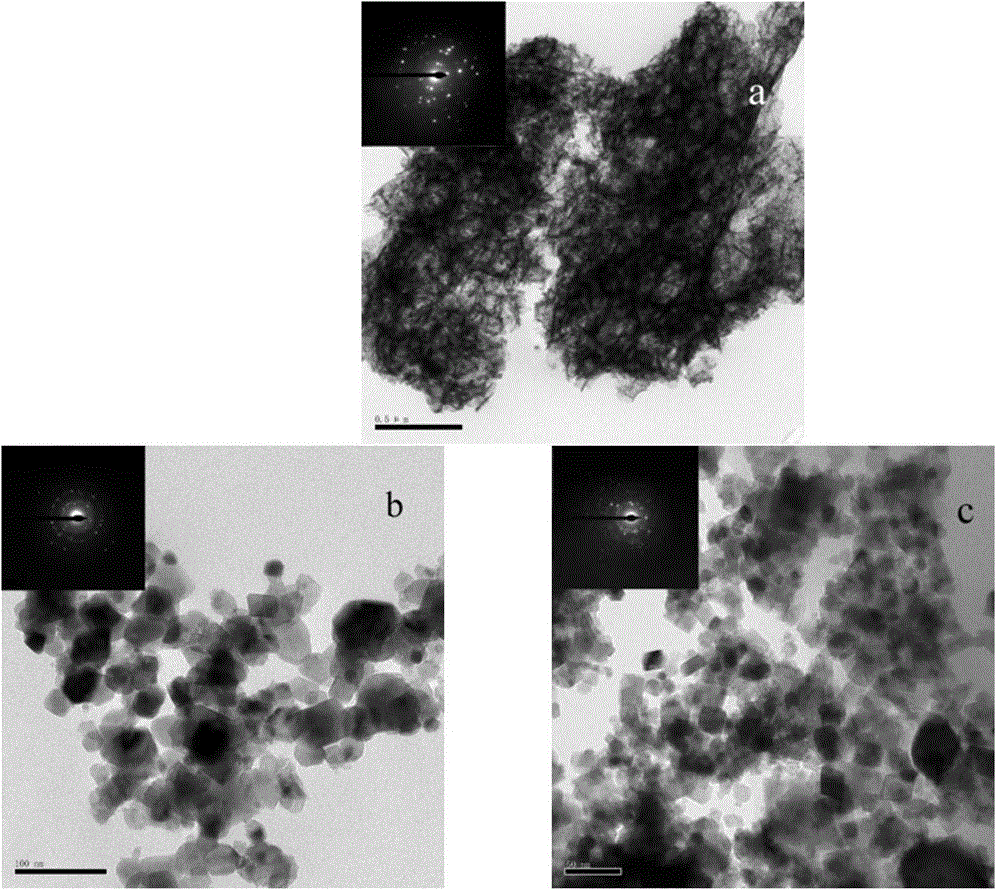
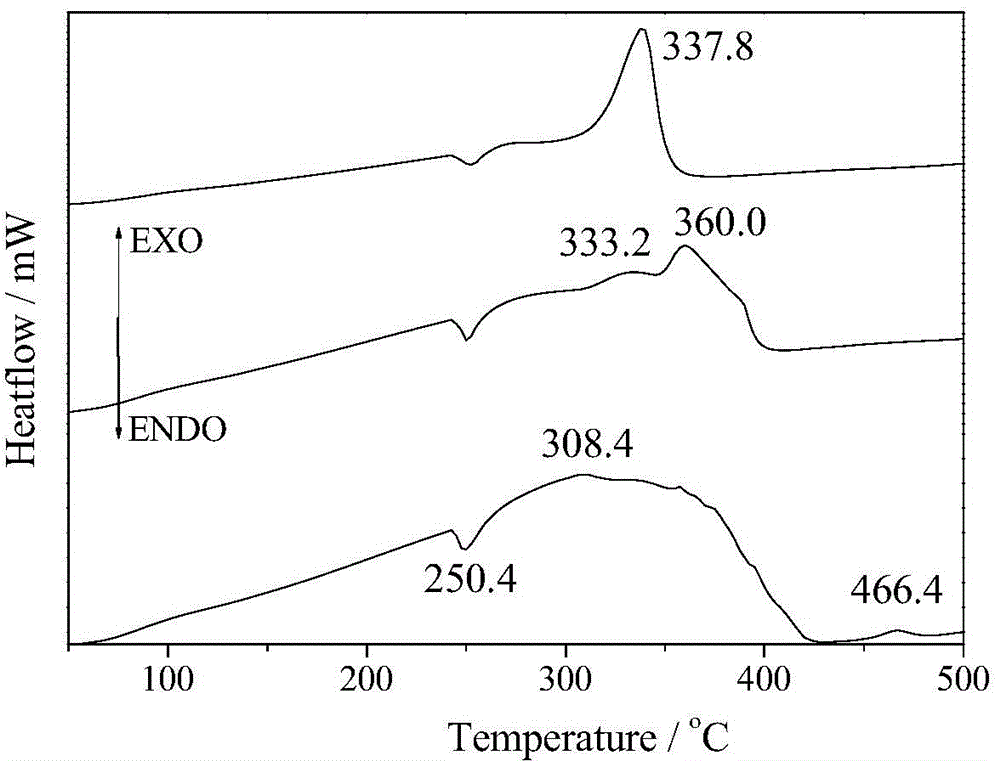
[0033] Dissolve 0.01mol ferric nitrate and 0.005mol magnesium nitrate in 100ml distilled water, add appropriate amount of glycine (molar ratio of glycine / metal salt Gly / M=[1, 2.5 or 3]:1) to form a mixed solution. Place it on a magnetically controlled temperature heater and react at 60°C for 2h. Raise the temperature to 110°C and evaporate the solution. After the evaporation is complete, the resulting gel will expand, and then release gas, and a self-propagating combustion reaction will occur to form a loose powder. The product was washed with hot deionized water and absolute ethanol, and filtered. Finally, dry at 60°C to obtain the final product. According to the XRD analysis of the product, the optimal dosage of glycine is 0.0375 mol (Gly / M=2.5).
Embodiment 2
[0035] Dissolve 0.01 mol of ferric nitrate and 0.005 mol of magnesium nitrate in 100 ml of distilled water, add 0.0375 mol of glycine and an appropriate amount of KCl (the molar ratio of KCl / metal salt KCl / M=2 / [3, 1 or 2]) to form a mixed solution. Place it on a magnetically controlled temperature heater and react at 60°C for 2h. Raise the temperature to 110°C and evaporate the solution. After the evaporation is complete, the resulting gel will expand, and then release gas, and a self-propagating combustion reaction will occur rapidly to form a loose powder. The product was washed with hot deionized water and absolute ethanol, and filtered. Finally, dry at 60°C to obtain the final product. According to the XRD analysis of the product, the optimal amount of KCl is 0.01 mol (KCl / M=2 / 3).
Embodiment 3
[0037] Dissolve 0.01mol ferric nitrate and 0.005mol magnesium nitrate in 100ml distilled water, add 0.0375mol glycine and appropriate amount of NaCl (NaCl / metal salt molar ratio NaCl / M=2 / [3, 1 or 2]) to form a mixed solution. Place it on a magnetically controlled temperature heater and react at 60°C for 2h. Raise the temperature to 110°C and evaporate the solution. After the evaporation is complete, the resulting gel will expand, and then release gas, and a self-propagating combustion reaction will occur rapidly to form a loose powder. The product was washed with hot deionized water and absolute ethanol, and filtered. Finally, dry at 60°C to obtain the final product. Through XRD analysis of the product, it was determined that the optimal amount of NaCl was 0.015 mol (NaCl / M=1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com