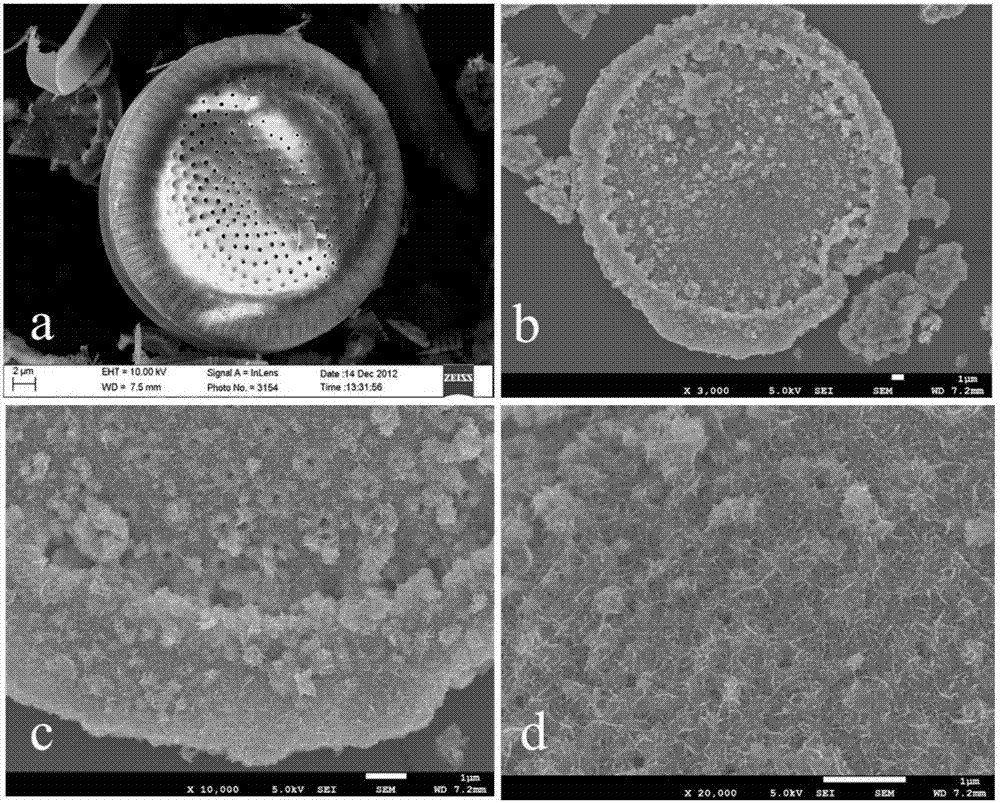
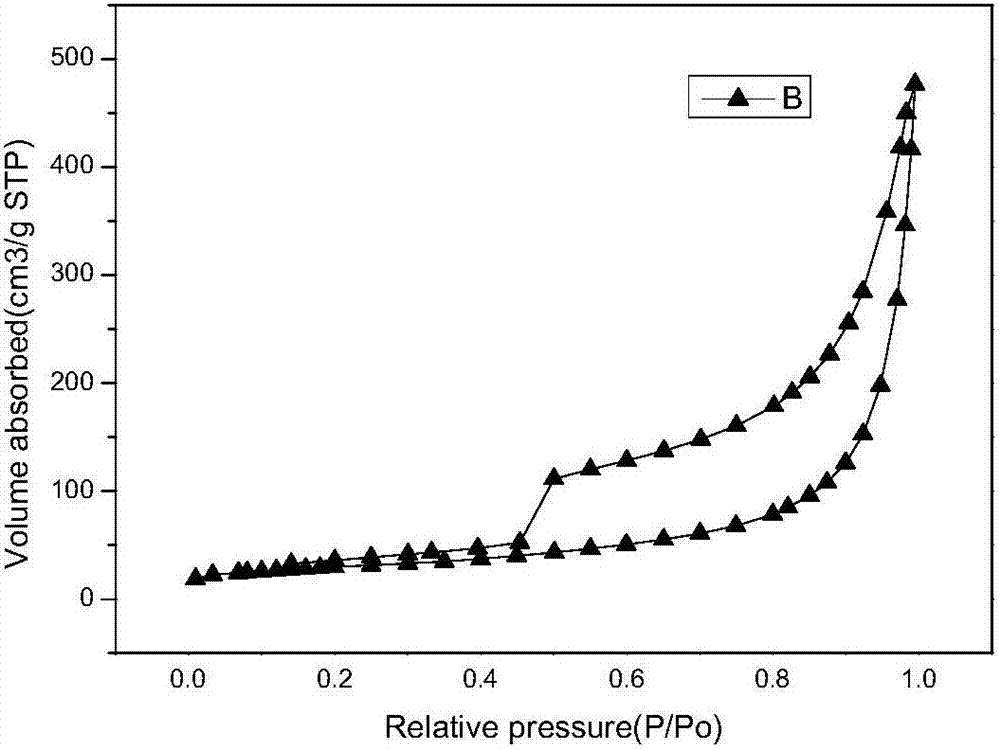
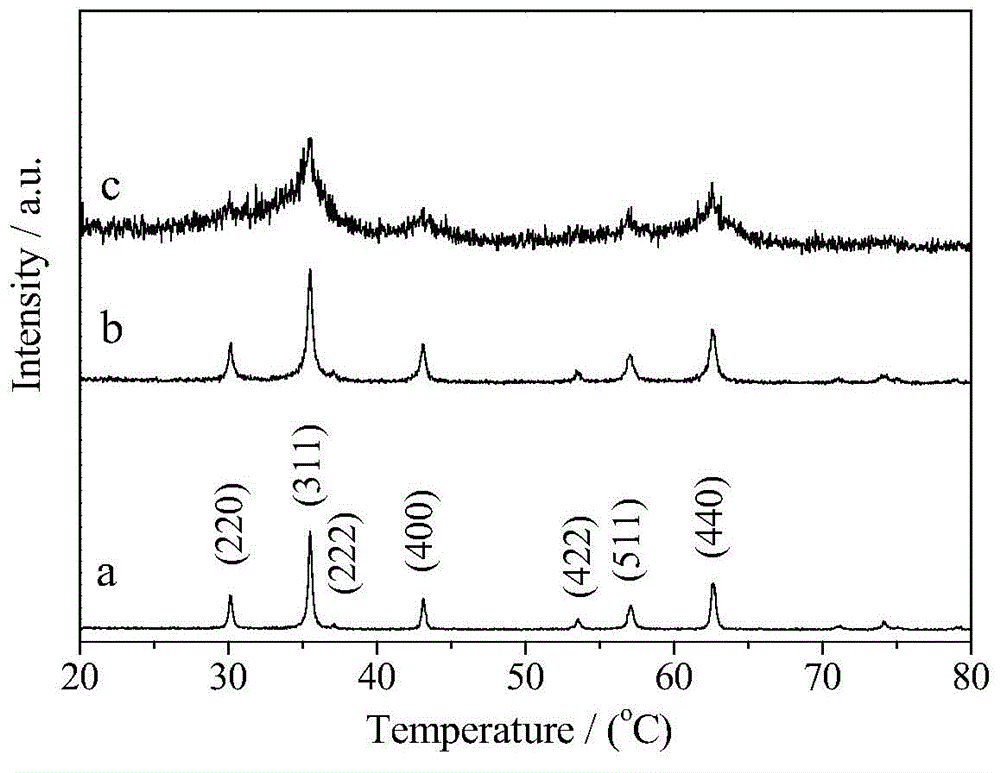
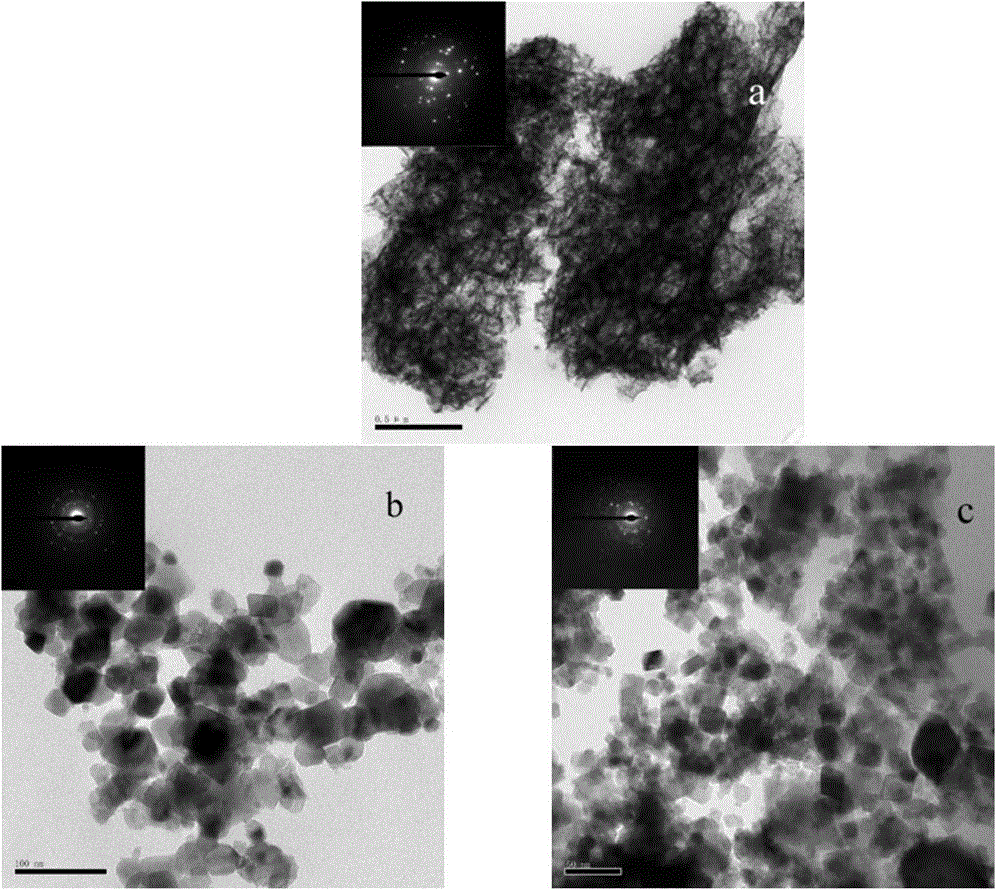
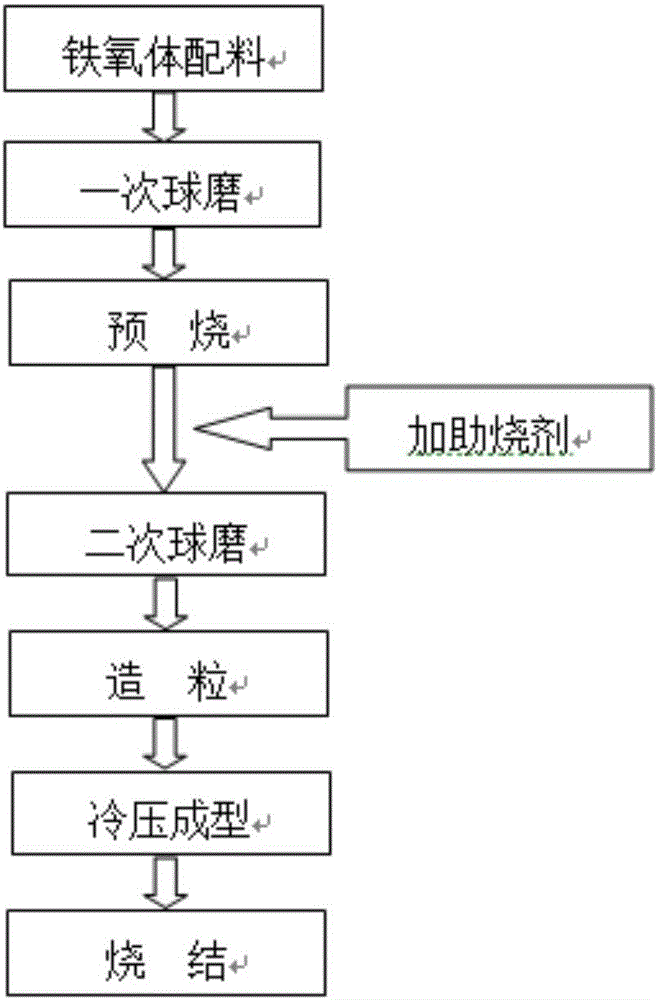
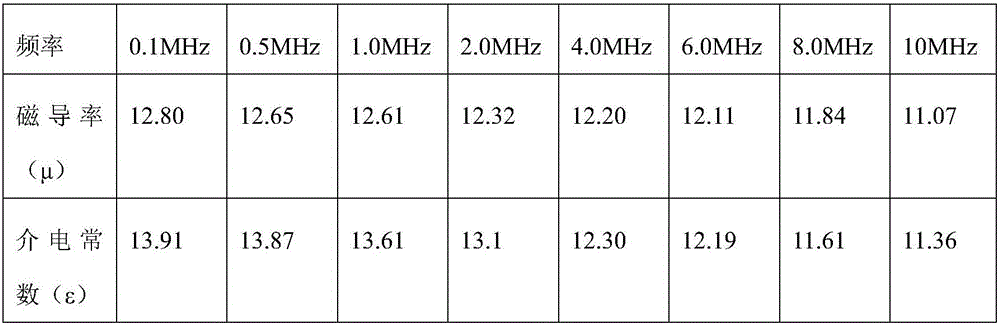
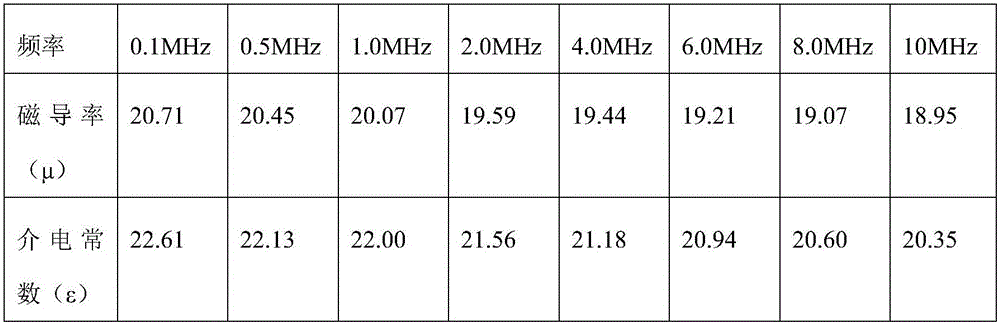
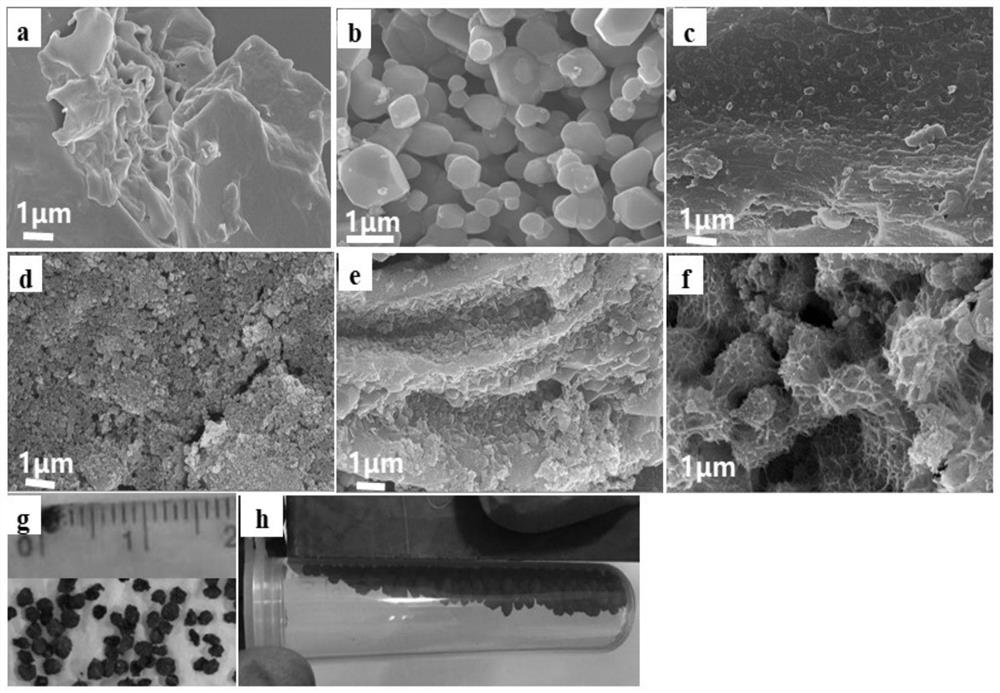
Patents
Literature
60 results about "Magnesium ferrite" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
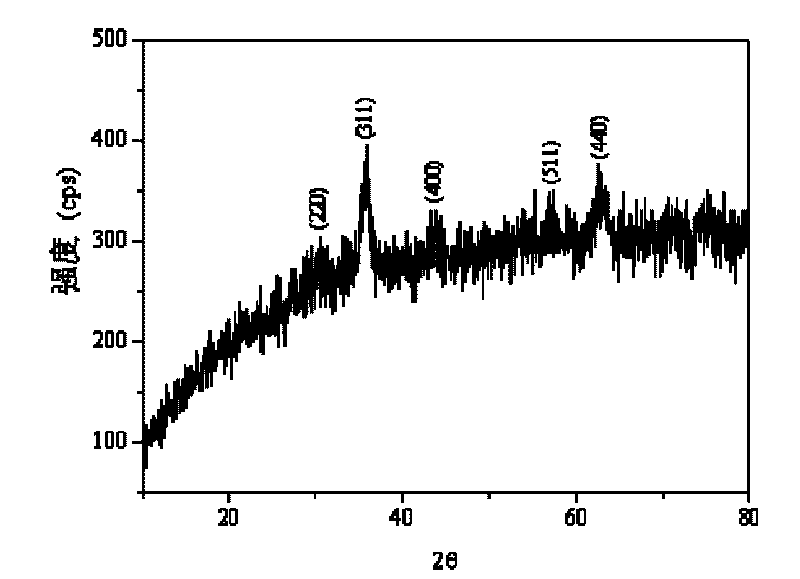
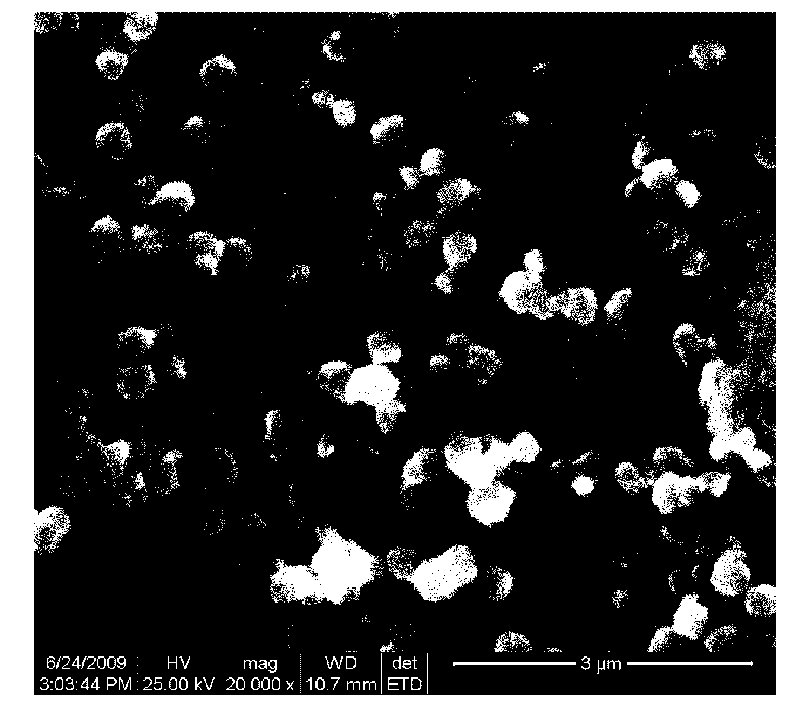
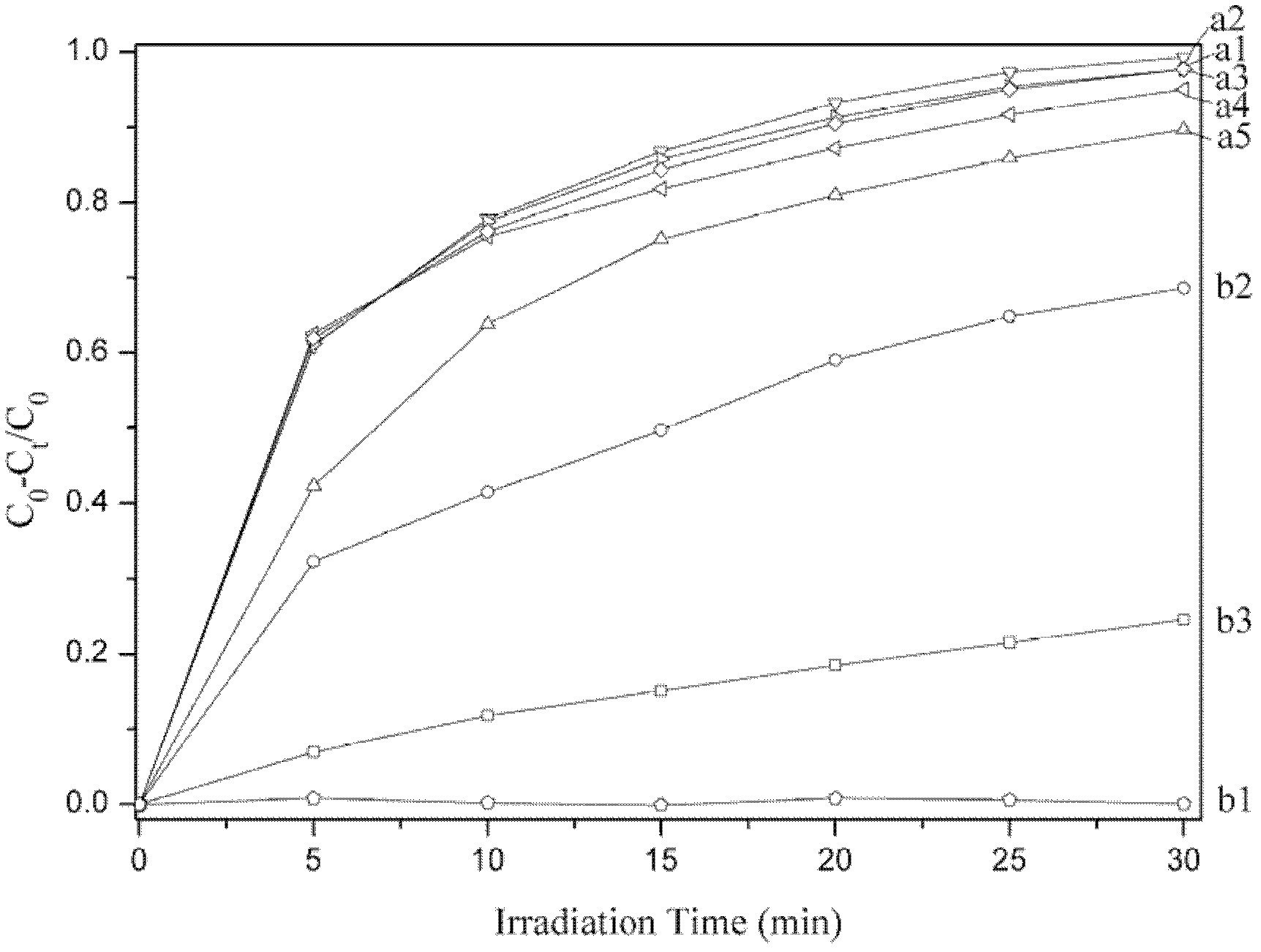
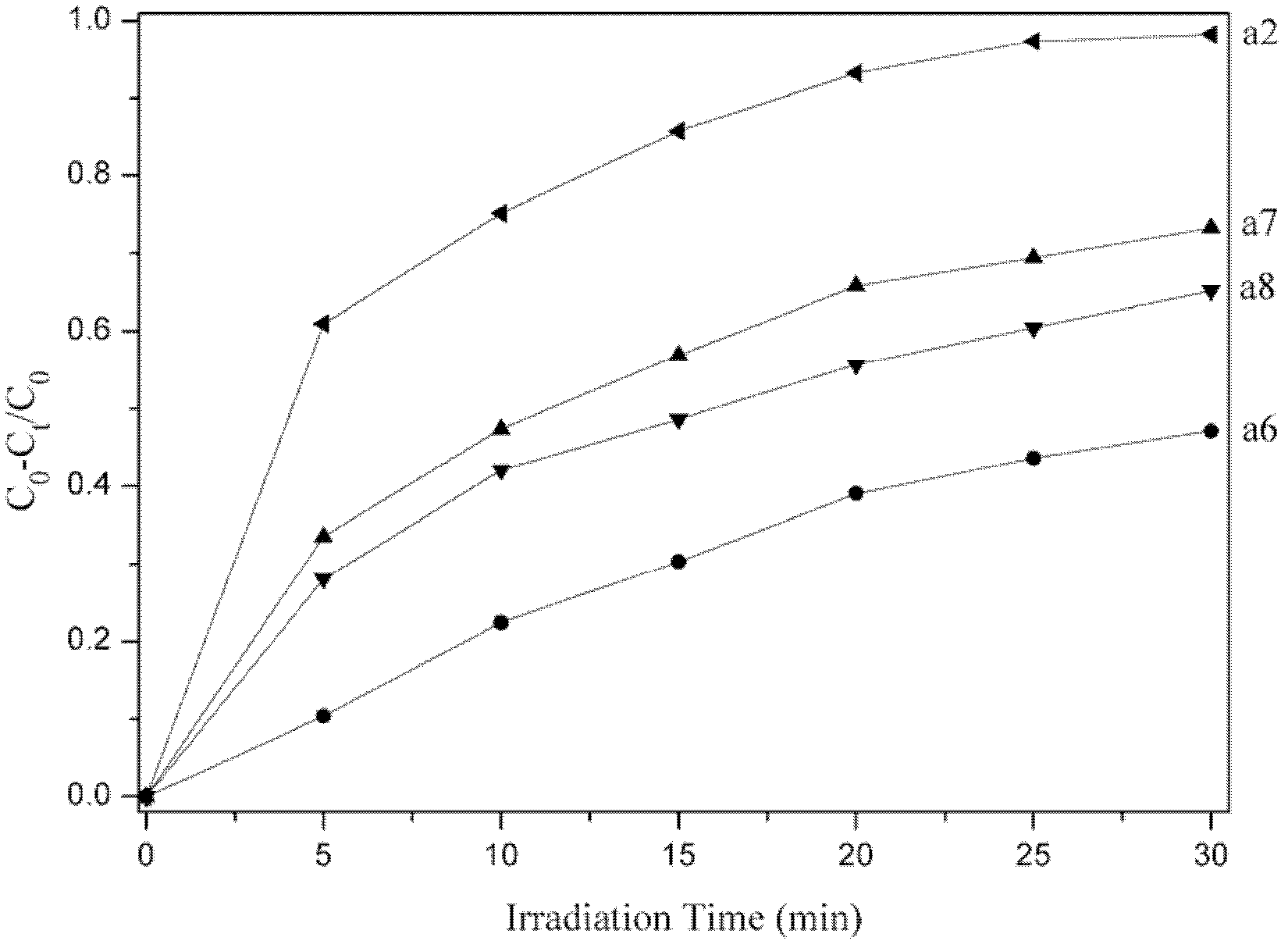
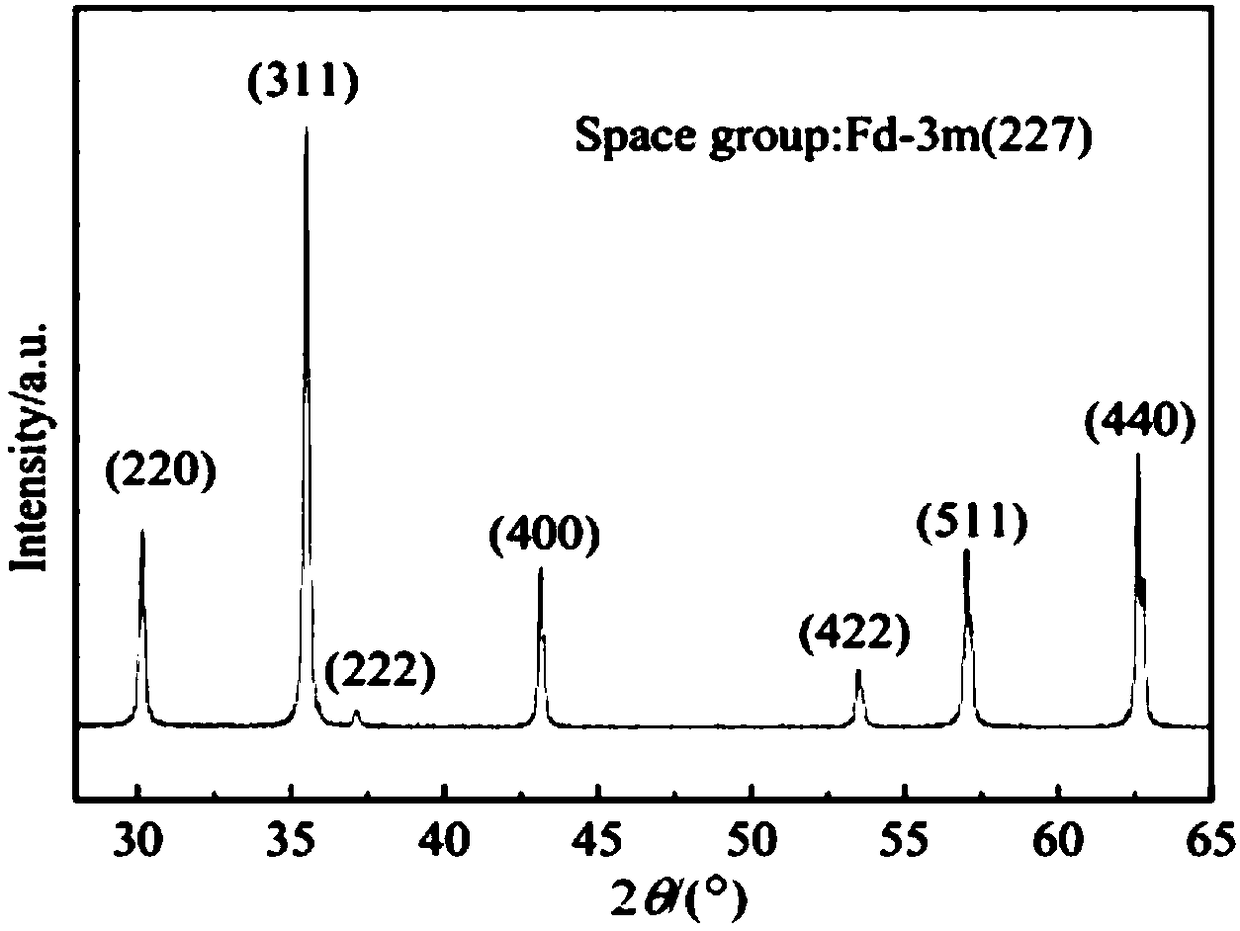
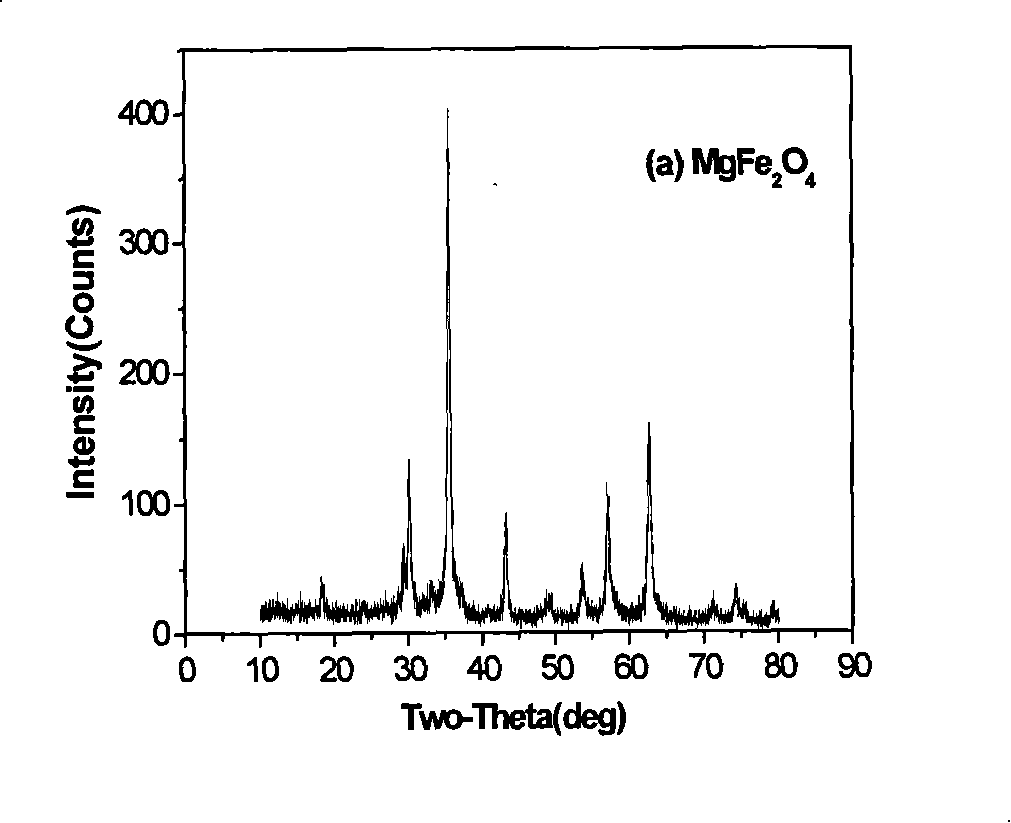
Spinel ferrites with the general formula AFe2O4 (A = Mn, Co, Ni, Mg, or Zn) are very important magnetic materials because of their interesting magnetic and electrical properties with chemical and thermal stabilities [1]. Magnesium ferrite (MgFe2O4) is one of the most important ferrites.
Composition for mobile phone case and method of manufacturing mobile phone case using the same
InactiveUS20100171234A1Low absorption rateReduce lossesCeramic shaping apparatusSolid electrolyte fuel cellsPolytetramethylene terephthalateAcrylic resin
There are provided a composition for mobile phone cases and a method of manufacturing a mobile phone case using the same. The composition comprises 70 to 97% by weight of a thermoplastic resin selected from the group consisting of polycarbonate (PC), acrylonitrile-butadiene-styrene (ABS), polybutyrene terephtalate (PBT), acrylic resin and combinations thereof; and 3 to 30% by weight of ferrite selected from the group consisting of nickel-zinc ferrite, manganese-magnesium ferrite, manganese-zinc ferrite, copper-zinc ferrite, manganese-magnesium-aluminum ferrite, yttrium iron garnet (YIG) ferrite and combinations thereof, wherein the composition is a pellet resin formed by extruding the thermoplastic resin and the ferrite at a high temperature of 160 to 290° C.
Owner:SAMSUNG ELECTRO MECHANICS CO LTD
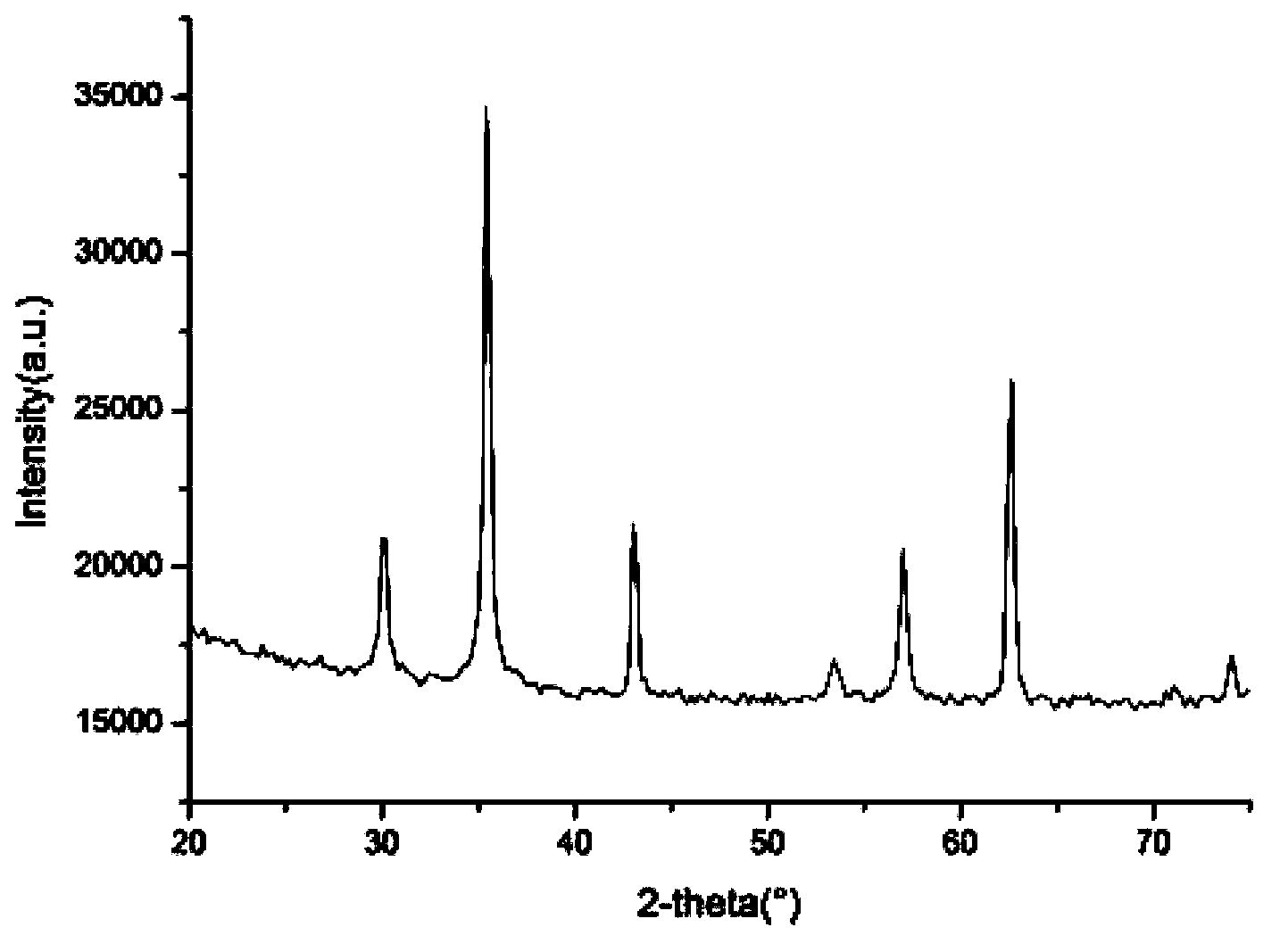
Preparation method of nano magnesium ferrite
InactiveCN102107910ALow costHigh purityNanotechnologyIron compoundsMegasonic cleaningCoprecipitation
The invention relates to a preparation method of nano magnesium ferrite, which comprises the following steps: mixing FeCl3.6H2O and FeCl2.4H2O in a mol ratio of 1:1, dropwisely adding a 2 mol / L NaOH solution, and preparing magnetic Fe3O4 nanoparticles by a chemical coprecipitation process; putting the magnetic Fe3O4 nanoparticles into a muffle furnace, and roasting at 500 DEG C for 3-4 hours to obtain nano Fe2O3; putting magnesium powder in a beaker, adding deionized water, and putting the beaker in an ultrasonic cleaner to treat for 5-8 hours, thereby obtaining a white turbid liquid; putting the supernatant of the turbid liquid in a clean beaker, drying in a drying box at constant temperature, and grinding the product to obtain Mg(OH)2; roasting the nano Mg(OH)2 in a muffle furnace at 350 DEG C for 3-4 hours, and roasting the Fe3O4 nanoparticles in a muffle furnace at 500 DEG C for 3-4 hours; respectively obtaining nano MgO and nano Fe2O3; and taking the nano Fe2O3 and the nano MgO, mixing the nano Fe2O3 and the nano MgO in a mol ratio of 1:1, adding deionized water, treating in an ultrasonic cleaner for 10-12 hours to obtain a red turbid liquid, sucking the supernatant of the turbid liquid into a clean beaker, drying the beaker in a drying box at constant temperature, and grinding the product to obtain the nano magnesium ferrite. The nano magnesium ferrite prepared by the method provided by the invention has the advantages of low cost, high purity, uniform appearance, environmental protection and no pollution.
Owner:UNIV OF SHANGHAI FOR SCI & TECH
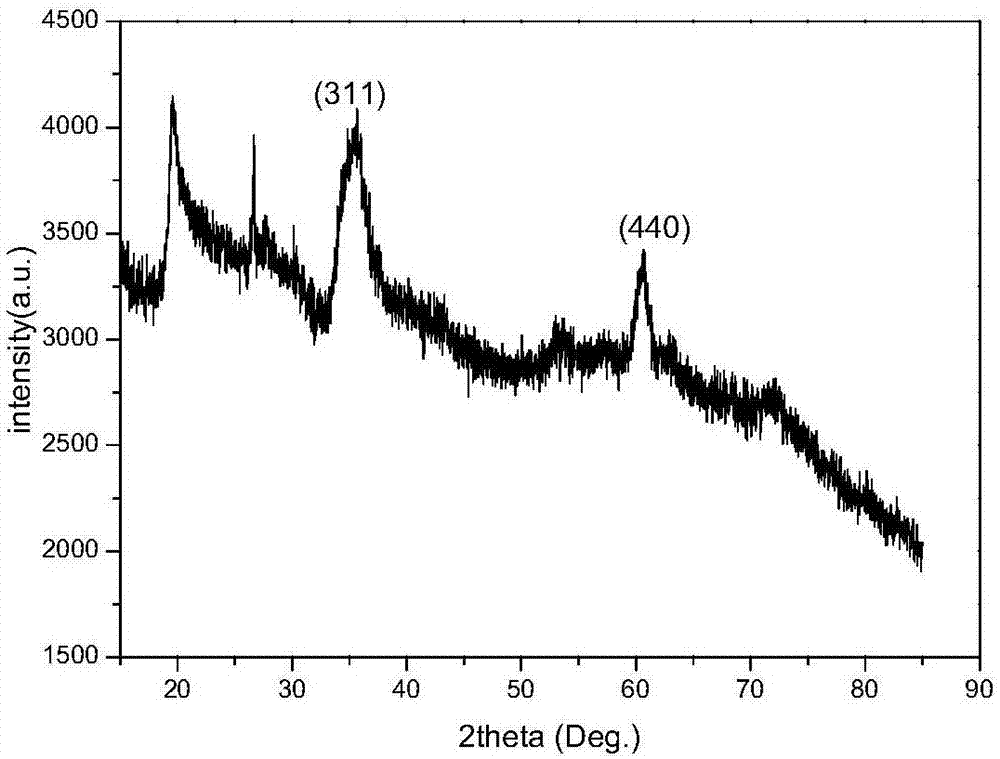
Method for synthesizing spinel structure magnesium frrite nano particles
InactiveCN101070192ALow costSimple processMagnesium compoundsIron compoundsAutoclaveMagnesium ferrite
Owner:TIANJIN UNIV
Preparation method for spherical nanometre magnesium ferrite desulfurizer
InactiveCN101708423AEasy to shapeImprove adsorption capacityOther chemical processesDispersed particle separationShape changeHigh pressure
The invention discloses a preparation method for a spherical nanometre magnesium ferrite desulfurizer, which belongs to the field of pollution control and technology. The method comprises the following steps of: dissolving a ferric nitrate and a magnesium nitrate in a glycol; adding an ammonium acetate into the mixture under the condition of violent stirring; putting the microemulsion in a high-pressure reactor to perform crystallization at 108 to 200 DEG C for 24 to 48h; and after filtering, washing and drying, calcining in a muffle furnace at 500 DEG C for 2h to obtain the spherical nanometre magnesium ferrite desulfurizer. The material is tested by an absorption test and the obtained magnesium ferrite has SO2 adsorbability. The method not only broadens a good magnesium shape changing and synthesizing method, but also has good SO2 adsorbability. The preparation method has good application value and application prospect in the field of absorption catalysis.
Owner:DALIAN UNIV OF TECH
Photocatalyst for degrading organic dye waste water pollutants and preparation method thereof
InactiveCN102600865AHigh catalytic activityImprove visible light absorptionWater/sewage treatment by irradiationWater contaminantsChemical compositionOrganic dye
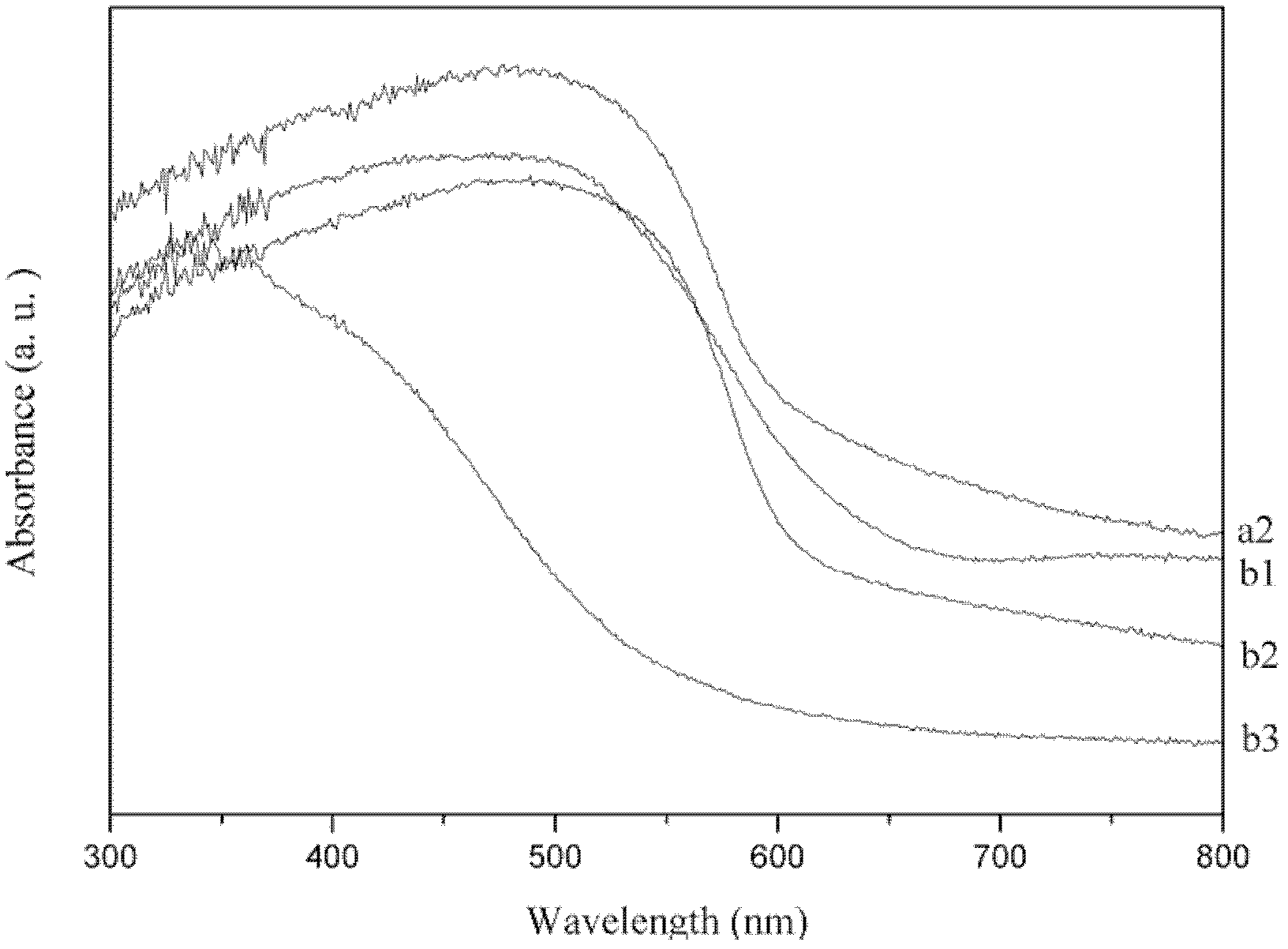
The invention relates to a photocatalyst for degrading organic dye waste water pollutants. The catalyst is formed by compounding magnesium ferrite and silver vanadate nanoparticles. The chemical composition general formula of the photocatalyst is mMgFe2O4 / Ag3VO4, wherein m is the mass ratio of MgFe2O4 to Ag3VO4; and m is more than 0 and less than or equal to 0.005. A preparation method of the photocatalyst comprises the following steps of: firstly, preparing magnesium ferrite and silver vanadate powder with a citric acid sol-gel method and a chemical precipitation method respectively; secondly, mixing and grinding the magnesium ferrite and silver vanadate powder for 10 minutes according to the mass ratio of the magnesium ferrite to the silver vanadate; and lastly, baking at the temperature of 200-500 DEG C, and baking for 4 hours to obtain a finished catalyst. The catalyst has a simple preparation method, has high visible light degrading performance on organic pollutants in dye waste water, and contributes to recycling simultaneously.
Owner:ZHEJIANG NORMAL UNIVERSITY
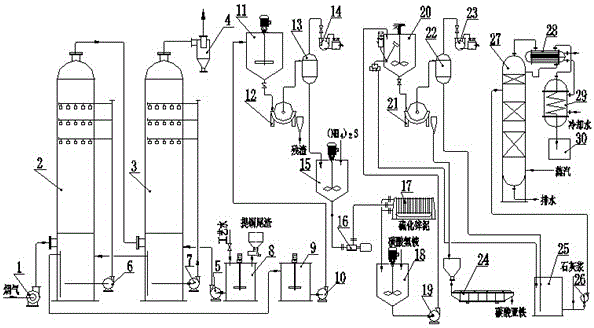
Method and device for removing SO2 in flue gas with copper extracting tailings and recycling copper extracting tailings
ActiveCN106422716AWith spray densityIncrease contact areaGas treatmentDispersed particle separationMetasilicateAmmonium sulfide
The invention relates to a method and device for removing SO2 in flue gas with copper extracting tailings and recycling the copper extracting tailings, and belongs to the technical field of environmental protection. Ferrous metasilicate (Fe2SiO4), magnesium ferrite (MgFe2O4), hedenbergite (CaFeSi2O6) and other sulfur removal active substances in the copper extracting tailings are used for reacting with SO2 in the flue gas in an aqueous solution to generate sulfite, then sulfate is generated under the action of oxygen in the flue gas, the SO2-containing flue gas makes contact with copper extracting tailing slurry, SO2 is absorbed, and thus the flue gas is purified; by adding ammonium sulfide, ammonium bicarbonate and lime milk step by step, iron ions, zinc ions, copper ions, ammonium ions and other ions in the sulfur removal slurry can be resourcelized to be reused.
Owner:KUNMING UNIV OF SCI & TECH
Preparation method of magnesium ferrite nano-particles
InactiveCN103420428AReduce preparation energy consumptionLow costMaterial nanotechnologyIron compoundsCoprecipitationColloid
The invention discloses a preparation method of magnesium ferrite nano-particles. The preparation method comprises the following steps of mixing magnesium powder and deionized water into a first mixture according to a ratio of 1g: 25mL to 1g: 20mL, carrying out ultrasonic hydrolysis of the first mixture at a normal temperature under normal pressure to obtain white liliquoid, carrying out constant-temperature drying of the white liliquoid, grinding the dried white liliquoid to obtain Mg(OH)2 nano-particles, weighing the Mg(OH)2 nano-particles and FeCl3.6H2O according to an element content ratio of Mg to Fe of 1: 2, adding the weighed Mg(OH)2 nano-particles into a first beaker with deionized water, carrying out ultrasonic pre-treatment for 1h, putting the weighed FeCl3.6H2O into a second beaker, carrying out precipitation by a chemical coprecipitation method to obtain Fe(OH)3 precipitates, diluting the Fe(OH)3 precipitates by deionized water so that the Fe(OH)3 precipitates have a pH value of 7, mixing the pretreated Mg(OH)2 and the dilute Fe(OH)3 precipitates having a pH value of 7 by stirring for a certain time to obtain a second mixture, carrying out ultrasonic activation of the second mixture under normal pressure, in ultrasonic activation, taking out the mixture, carrying out stirring multiple times to obtain a third mixture, carrying out constant-temperature drying of the third mixture to obtain a forth mixture, grinding the forth mixture into powder, putting the powder into a muffle furnace, carrying out calcination at a temperature of 700 DEG C for 5h, and carrying out cooling in the muffle furnace.
Owner:UNIV OF SHANGHAI FOR SCI & TECH
Preparation method of diatomite/magnesium ferrite composite material
ActiveCN107469765AIncrease surface areaLow priceOther chemical processesWater treatment compoundsNanostructureIon
The invention relates to a preparation method of a diatomite / magnesium ferrite composite material which can adsorb and degrade hexavalent chromium ions. The method comprises the following steps: 1) dissolving diatomite into ammonia water, and fully dipping the diatomite with the ammonia water by means of stirring so as to form diatomite suspension; 2) adding hexadecyl trimethyl ammonium bromide into the suspension to obtain a mixture and evenly stirring the mixture; 3) dropwise adding a solution containing magnesium chloride and ferrous oxalate into the evenly-stirred suspension slowly to obtain a mixed solution; 4) carrying out aging treatment on the mixed solution for 8 to 10 hours under a hydrothermal condition of 160 to 200 DEG C, cooling, washing, filtering and drying to obtain the diatomite / magnesium ferrite composite material. According to the method disclosed by the invention, magnesium ferrite with an ordered nanostructure is loaded on a diatomite algae disc, so that the limitation during application of a nanostructure material can be effectively solved, the adsorption efficacy of the diatomite also can be improved, and the catalytic reduction ability of the composite material is given to the hexavalent Cr at the same time.
Owner:BEIJING UNIV OF TECH
Environmentally friendly refractory material and preparation method thereof
The invention relates to an environmentally friendly refractory material. The environmentally friendly refractory material comprises, by mass, 60-80 parts of magnesia particles, 10-20 parts of magnesia fine powder, 10-30 parts of silicon carbide particles, 10-30 parts of silicon powder, 10-20 parts of magnesium ferrite, 14-20 parts of expanded perlite, 10-22 parts of chrome oxide fine powder, 10-20 parts of alumina silicate fibers, 12-24 parts of aluminum nitride, 10-24 parts of potassium feldspar, 12-20 parts of mullite, 14-20 parts of carbon black, 16-20 parts of chrome oxide micro-powder, 12-20 parts of tabular corundum powder, 1-10 parts of an anti-oxidant and 4-10 parts of an organic bonding agent. The environmentally friendly refractory material has high strength, thermal shock resistance, oxidation resistance, wear resistance, erosion resistance, high temperature resistance and long service life.
Owner:HUANGHE S & T COLLEGE
Preparation method of high-specific surface nanometer magnesium ferrite catalyst material capable of being used in solid propellant
ActiveCN104353461AGood dispersionHigh crystallinityMaterial nanotechnologyIron compoundsIron saltsHigh energy
The invention discloses a preparation method of a high-specific surface nanometer magnesium ferrite catalyst material capable of being used in a solid propellant, relating to the field of nanometer material synthesis and high-energy solid propellants. The preparation method comprises the following steps: dissolving an inorganic iron salt and an inorganic magnesium salt in distilled water, adding glycine and an inert inorganic salt, reacting at a temperature of 45-75 DEG C to form a wet sol, raising the temperature to 95-125 DEG C to evaporate a solution after reaction, rapidly performing self-propagating combustion reaction on dried gel obtained after the wet gel is completely evaporated so as to generate powder, washing and drying the dry powder to obtain the nanometer MgFe2O4 catalyst material. The prepared high-specific surface nanometer magnesium ferrite catalyst material is high in catalysis activity; the preparation method is simple and controllable in process, free from pollution, and low in cost, thereby having great application prospect in the field of solid propellants; the high-specific surface nanometer magnesium ferrite catalyst material is easily produced in a large scale.
Owner:南京美材科技有限公司
Synthesis method and application of magnesium ferrite/molybdenum sulfide heterostructure nanowires
ActiveCN105289660AGood chemical stabilityImprove photoelectrochemical performancePhysical/chemical process catalystsHydrogen productionNanowirePhysical chemistry
The invention belongs to the technical field of nanomaterial synthesis. Firstly, an electrostatic spinning method is adopted to synthesize magnesium ferrite, then an evaporation method is adopted to enable molybdenum sulfide to grow on the magnesium ferrite so as to form the magnesium ferrite / molybdenum sulfide heterostructure nanowires. The tetracycline degradation rate of the prepared magnesium ferrite / molybdenum sulfide heterostructure nanowires prepared by means of the synthesis method is up to 92% in 120 minutes, and the hydrogen production efficiency in a water and methanol mixed solution is 5.8 mmol / h.m<2>.
Owner:常熟市明远达无纺包装材料有限公司
Lithium sulfur battery composite cathode material and preparation method thereof
ActiveCN108054350AMature technologySimple processCell electrodesSecondary cellsChemical adsorptionSulfur utilization
The invention relates to a lithium sulfur battery composite cathode material and a preparation method thereof. The composite cathode material is prepared by compounding ferrite with elemental sulfur.The ferrite is one of magnesium ferrite, zinc ferrite, copper ferrite or manganese ferrite. The preparation method includes: preparing a ferrite material by high temperature calcination method, and then conducting compounding with elemental sulfur by liquid phase method. The preparation method has a mature process and simple procedure, and is easy to acquire a high sulfur content composite cathodematerial. The lithium sulfur battery composite cathode material prepared by the invention utilizes the strong chemical adsorption effect of ferrite on polar lithium polysulfide, greatly inhibits thedissolution of lithium polysulfide in an ether electrolyte solution, accordingly slows down the shuttle effect, and then shows characteristics of high sulfur content, high sulfur utilization and highcycle stability.
Owner:NANKAI UNIV
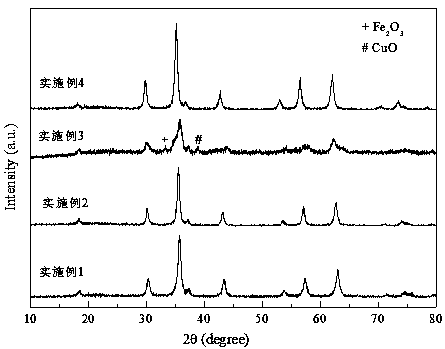
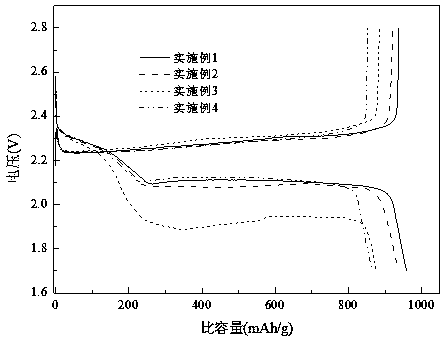
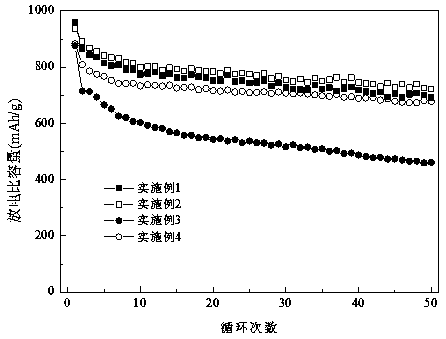
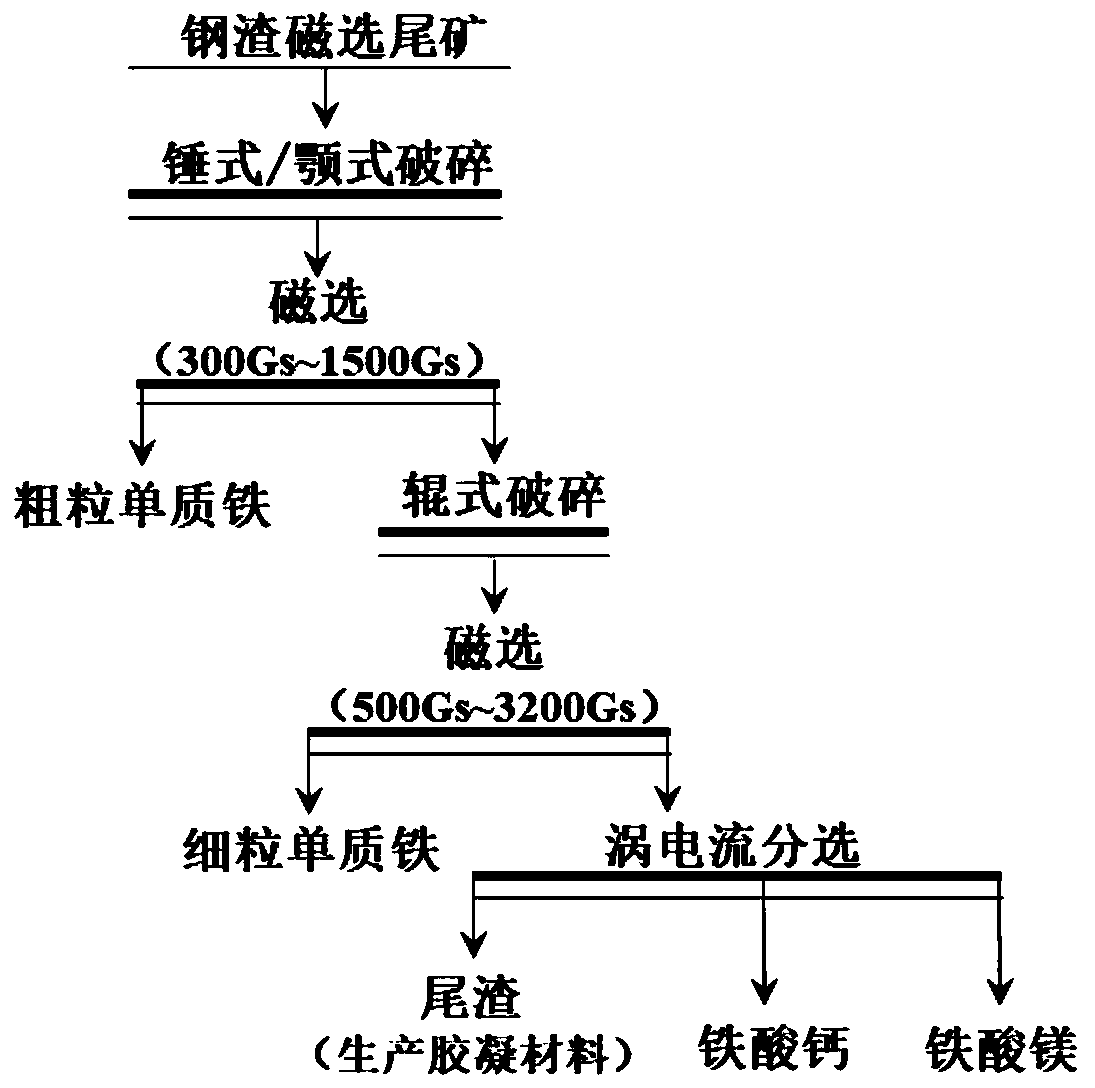
Method for separating calcium ferrite and magnesium ferrite from steel slag magnetic separation tailings
The invention belongs to the field of resource regeneration, and particularly relates to a method for separating calcium ferrite and magnesium ferrite from steel slag magnetic separation tailings. Themethod comprises the following steps: firstly, crushing the steel slag magnetic separation tailings containing 20%-30% of iron to 10mm-13mm by using selective crushing equipment, and selectively dissociating coarse-grained elemental iron particles; secondly, conducting magnetic separation under the field intensity of 300 Gs-1500 Gs, removing coarse-grained elemental iron in the form of magnetic concentrate and using the coarse-grained elemental iron as a steelmaking raw material; thirdly, finely crushing the magnetic separation tailings to 0.5mm-3.5mm by using roller type crushing equipment,and selectively dissociating calcium ferrite and magnesium ferrite by using hardness difference; and fourthly, carrying out magnetic separation under the field intensity of 500 Gs-3200 Gs, separatingout fine-grained elemental iron and merging the fine-grained elemental iron into the steelmaking raw material, and purifying residual calcium ferrite and magnesium ferrite crude products through a vibration bed eddy current sorting machine, thus obtaining a final product. The tailings without specific purposes generated in the process are merged for producing a cementing material, and finally all-component resource utilization of the steel slag is achieved.
Owner:UNIV OF SCI & TECH BEIJING
Molybdenum sulfide-ferrite nano-enzyme as well as preparation and application
ActiveCN108046331ALarge specific surface areaMany active sitesPhysical/chemical process catalystsIron compoundsSodium acetateHydrazine compound
The invention relates to a molybdenum sulfide-ferrite nano-enzyme as well as a preparation method and an application method thereof. The preparation method comprises the following steps: uniformly mixing ferric chloride hydrate, magnesium chloride hydrate and dodecyl amine with a proper amount of ethylene glycol; enabling the components to react in a high-pressure reaction kettle, and repeatedly cleaning the product; drying the product so as to obtain ferrite magnesium; dissolving ammonium tetrathiomolybdate into dimethyl formamide; slowly adding hydrazine hydrate, and uniformly mixing; putting a proper amount of the ferrite magnesium into the mixed liquid; enabling the components to react in the high-pressure reaction kettle, and repeatedly cleaning the product; drying the product so as to obtain molybdenum sulfide-ferrite magnesium; putting the molybdenum sulfide-ferrite magnesium into a proper amount of TMB (Tetramethylbenzidine) and hydrogen peroxide-sodium acetate buffer solutionsof different concentrations; culturing, and testing the concentration of hydrogen peroxide. Results show that when being adopted to detect hydrogen peroxide, the molybdenum sulfide-ferrite magnesiumnano-enzyme is convenient and rapid to operate, high in sensitivity and wide in detection concentration range.
Owner:YANGZHOU UNIV
Preparation method of magnesium ferrite/silver phosphate compound photocatalyst
ActiveCN104028287AEasy transitionAvoid recombinationPhysical/chemical process catalystsWater/sewage treatment by irradiationSilver phosphateSodium hydroxide
The invention discloses a preparation method of a magnesium ferrite / silver phosphate compound photocatalyst. The preparation method comprises the following steps of (1) dropwise adding 50-100mL of 0.2-0.4mol / L silver nitrate in 50-100mL of 0.1-0.2mol / L sodium hydroxide solution while stirring, and separating a supernate so as to obtain silver oxide particles; adding 30-50mL of an ammonia-water solution with the mass concentration being 20%-25%, and stirring until silver oxide is fully dissolved to generate a silver ammonia complexing solution; and adding 0.4-0.6mol of ferric nitrate and 0.2-0.3mol of magnesium nitrate to the silver ammonia complexing solution, after stirring for 3-4h, adding 0.02-0.03mol of sodium dihydrogen phosphate, unceasingly stirring for 2-3h, filtering and washing for 3-4 times, drying at 110-120DEG C, roasting for 5-6h at 500-600DEG C, and performing furnace cooling so as to obtain the magnesium ferrite / silver phosphate compound photocatalyst. The preparation method provided by the invention has the advantages that a synthetic process is simple, silver phosphate and magnesium ferrite are closely contacted, transition of photoproduction electrons is facilitated, the recombination is avoided, and the catalytic efficiency is improved.
Owner:南通豫湖机械有限公司
Formula of high temperature-resistant magnesium ferrite orange-yellow pigment and preparation process thereof
Owner:南通宝聚颜料有限公司
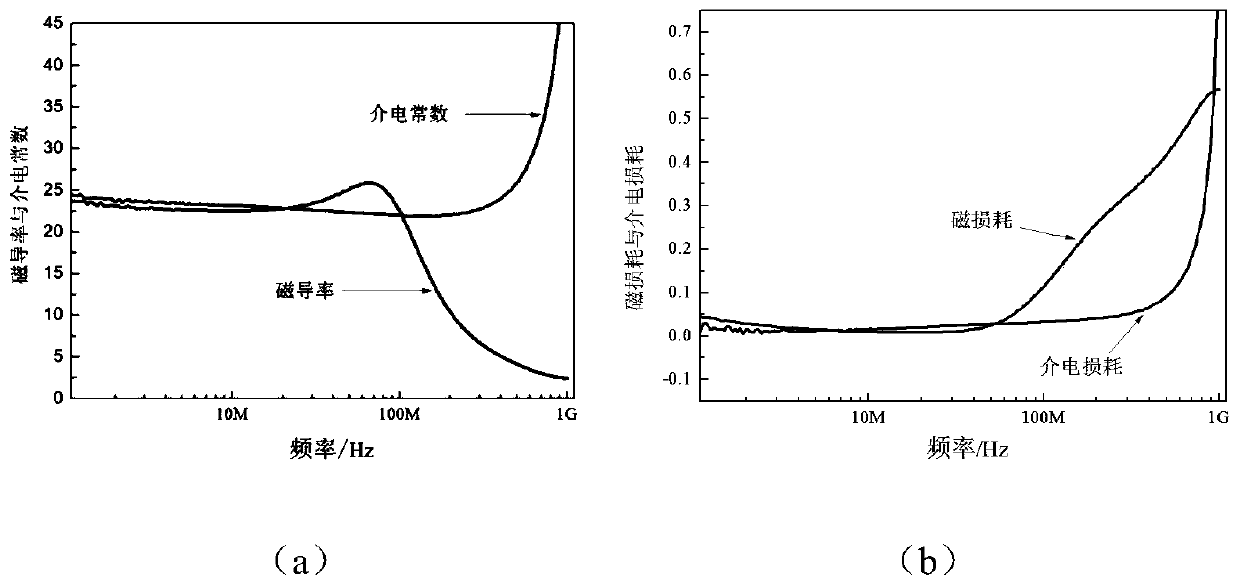
Magnesium ferrite-based low-loss magnetic dielectric material and preparation method thereof
The invention discloses a magnesium ferrite-based low-loss magnetic dielectric material and belongs to the field of electronic materials. The magnetic dielectric material is prepared from magnesium ferrite as a material through praseodymium doping modification, the praseodymium-doped magnesium ferrite material is a Mg1-xCdxFe2-yPryO4 spinel ferrite material, x is 0.1-0.3, and y is 0.02-0.08. According to the magnetic dielectric material disclosed by the invention, magnetic properties and dielectric properties of the material are adjusted through modification of Pr ions, low-temperature sintering and magnetic dielectric approximate equality are achieved, uniform crystal grain growth is controlled through a material synthesis process, and the material has low magnetic consumption parametersand has equal magnetic dielectric properties and low consumption within a frequency range of 1-30MHz; when being used as an antenna base plate material, the magnetic dielectric material is capable ofwell achieving miniaturization of an antenna, the radiation efficiency and the bandwidth of a microstrip antenna can be improved, and a novel scheme is provided for designing of small-size wireless communication equipment.
Owner:UNIV OF ELECTRONICS SCI & TECH OF CHINA
Preparation method of magnesium ferrite nanofiber negative electrode material
InactiveCN106159256ASimple preparation processLow costMaterial nanotechnologyCell electrodesFiberElectrospinning
The invention discloses a preparation method of a magnesium ferrite nanofiber negative electrode material, and belongs to the technical field of nanometer materials and chemical power supplies. The MgFe2O4 nanofiber negative electrode material is prepared through an electrostatic spinning and calcination technology, and the method is simple in preparation process, low in cost, friendly to the environment and beneficial to further production expansion. The lithium ion battery negative electrode material, namely MgFe2O4 nanofiber, has a high discharge platform, large initial discharge capacity and stable circulating performance, and has wide development prospects as a new generation of lithium ion battery negative electrode material.
Owner:JIANGNAN UNIV
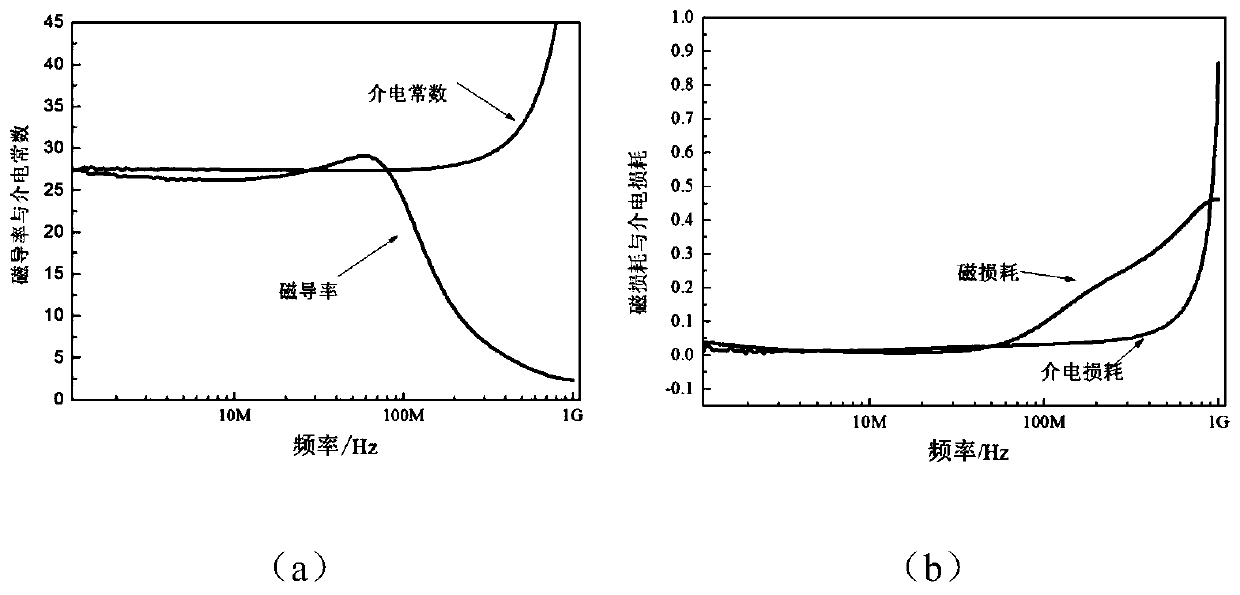
Magnesium ferrite-based magnetic dielectric material and preparation method therefor
ActiveCN106587976ALower sintering temperatureHigh magnetic permeability characteristicsRadiating elements structural formsDielectricMiniaturization
The invention discloses a magnesium ferrite-based magnetic dielectric material and a preparation method therefor, and belongs to the field of an electronic material. The magnesium ferrite-based magnetic dielectric material consists of a main phase material and an auxiliary phase material at a mass percentage ratio of 100 to (2-20) in a compounding manner, wherein the main phase material is Mg<1-x>Cd<x>Fe<2>O<4> spinel ferrite, x is equal to 0.1-0.3, and the auxiliary phase material is Bi<2>O<3>; and performing ball milling and mixing on the main phase material and the auxiliary phase material, and then carrying out drying, sieving, palletizing, pressing and shaping, and next, sintering at a temperature of 880-960 DEG C for 1-6h. The magnetic dielectric material disclosed by the invention realizes low-temperature sintering and magnetic dielectric approximate equality, and has equal magnetic dielectric property and low loss property at a frequency range of 0.1-10MHz; and when the magnetic dielectric material is used as an antenna substrate material, miniaturization of an antenna can be well realized, and the radiation efficiency and bandwidth of a microstrip antenna can be improved, so that a new scheme is provided for design of small-dimensional wireless communication equipment.
Owner:UNIV OF ELECTRONICS SCI & TECH OF CHINA
Magnetic magnesium ferrite biochar composite microsphere phosphorus removal adsorbent, and preparation method and application thereof
InactiveCN111701567APhosphorus adsorption performance is goodRaw materials are easy to getOther chemical processesWater contaminantsPhosphateCarbonization
The invention discloses a magnetic magnesium ferrite biochar composite microsphere phosphorus removal adsorbent, and a preparation method and application thereof. The magnetic magnesium ferrite biochar composite microsphere phosphorus removal adsorbent is prepared by using reed as a raw material and conducting magnesium ferrite modification, lanthanum alginate embedding and balling and high-temperature carbonization. A magnetic magnesium ferrite biochar composite microsphere prepared by the method has a diameter of 2.5-3.5 mm, has high adsorption capacity on phosphate, and is excellent in masstransfer performance and regeneration effect; magnetic magnesium ferrite modified biochar is wrapped in the porous microsphere, so the loss of nanometer magnesium ferrite is prevented, the mass transfer performance of the microsphere is improved, and meanwhile, the effective recovery and regeneration of the adsorbent are realized by utilizing the advantages of easy settling performance of the microsphere and the magnetism of the magnesium ferrite in the microspheres; and the preparation method has the advantages of easy availability of raw materials, simple technique and mild conditions, andis suitable for large-scale industrial production and use.
Owner:XI AN JIAOTONG UNIV
A kind of magnesium ferrite based magnetic dielectric material and preparation method thereof
ActiveCN106587976BLower sintering temperatureHigh magnetic permeability characteristicsRadiating elements structural formsMiniaturizationAntenna substrate
A magnesium ferrite-based magnetic dielectric material and a preparation method thereof belong to the field of electronic materials. The magnesium ferrite-based magnetic dielectric material is composed of a main phase material and an auxiliary phase material in a mass percentage ratio of 100: (2-20), and the main phase material is Mg 1‑x Cd x Fe 2 o 4 Spinel ferrite, the value of x ranges from 0.1 to 0.3, and the auxiliary phase material is Bi 2 o 3 The main phase material and the auxiliary phase material are mixed by ball milling, dried, sieved, granulated, pressed and formed, and then sintered at 880-960° C. for 1-6 hours. The magnetic dielectric material of the present invention realizes low-temperature sintering and magnetic dielectric approximately equal, and has equal magnetic dielectric properties and low loss in the frequency range of 0.1MHz to 10MHz; when the magnetic dielectric material is used as an antenna substrate material, it can well realize antenna The miniaturization of the microstrip antenna is conducive to improving the radiation efficiency and bandwidth of the microstrip antenna, which provides a new solution for the design of small-sized wireless communication equipment.
Owner:UNIV OF ELECTRONICS SCI & TECH OF CHINA
Catalyst for catalytic elimination of carbon monoxide and formaldehyde under normal temperature and humidity conditions and preparation method thereof
ActiveCN106391007BSolution to short lifeNo lossGas treatmentDispersed particle separationPlatinum oxideCalcium ferrite
The invention provides a catalyst for catalytic removal of carbon monoxide and formaldehyde under conditions of ambient temperature and humidity, and belongs to the technical field of catalyst for harmful gas. The catalyst is a supported noble metal catalyst formed by depositing an active component of noble metal on a spinel-structure oxide support containing an aid, wherein the spinel-structure oxide support is one or two of magnesium ferrite, calcium ferrite, strontium ferrite, barium ferrite, cobalt ferrite, nickel ferrite, copper ferrite, zinc ferrite and manganous ferrite; the active component of noble metal is elemental platinum or platinum oxide, and the aid is an oxide of sodium. The catalyst can catalyze carbon monoxide and formaldehyde for complete oxidation under the conditions of ambient temperature and humidity, and products only comprise carbon dioxide and water. The catalyst has higher stability, the activity cannot be reduced after the catalyst is stored for a long time, and the catalyst is not required to be reduced before use; the catalyst has the advantages of being easy to prepare, good in product repeatability, convenient to store and transport and the like and has quite good application prospect.
Owner:JILIN UNIV
Magnetism recyclable nano adsorbent, preparation method and application thereof
InactiveCN104587948AStable and excellent adsorptionSimple processMaterial nanotechnologyOther chemical processesCongo redMagnesium salt
The invention discloses a preparation method of a magnetism recyclable nano adsorbent. The preparation method comprises the following steps: dissolving a proper amount of zinc salt (or magnesium salt) into glycol, adding moderate ferric salt and NH4F to dissolve, uniformly stirring, performing solvothermal reaction on the obtained solution, washing and drying reaction product, placing in a muffle furnace to calcinate, and waiting for naturally cooing to obtain a zinc ferrite or magnesium ferrite powder adsorbent. The adsorbent prepared by the solvothermal method has dual adsorption capacity on organic dye Congo red and heavy metal Pb2+. The preparation cost is low, and the process is simple; the prepared adsorbent is stable and excellent in property and good in repeatability, and the magnetism is recyclable. The adsorbent material can be widely applied to governing aspects such as heavy metal pollution and organic dye pollution.
Owner:CHINA THREE GORGES UNIV
Prepn. of ferrite permanent-magnet materials
This invention relates to a method to prepare permanent ferromagnetic materials. It includes following steps: addition of adhesives into ferro oxide magnetic materials; moisture adjustment; molding, magnetization and compression of moisture-adjusted mixture and sintering at high temperatures. The characteristics is that, the ferro oxide magnetic materials are chosen from one of several species of ferro oxide, manganese-zincum ferrite, manganese ferrite, magnesium ferrite and copper-zincum ferrite, and metal nitrite weighing 1~2wt% of the total magnetic materials mentioned above is also added. The adhesives include thermoplastic resin containing keto-groups and weigh 0.1~0.2wt% of the total mixture. The moisture content in the mixture during moisture adjustment is 0.2~0.4wt%. Finally, the sintering is carried out at a temperature between 350~450 deg. C. This invention has the outstanding specificity that low-temperature sintering can be realized by modification of raw materials and adhesives and thus cost is cut down; orientation can be enhanced and magnetic properties can be optimized.
Owner:刘仁臣
Lanthanum-doped magnesium ferrite composite material and preparation method and application thereof
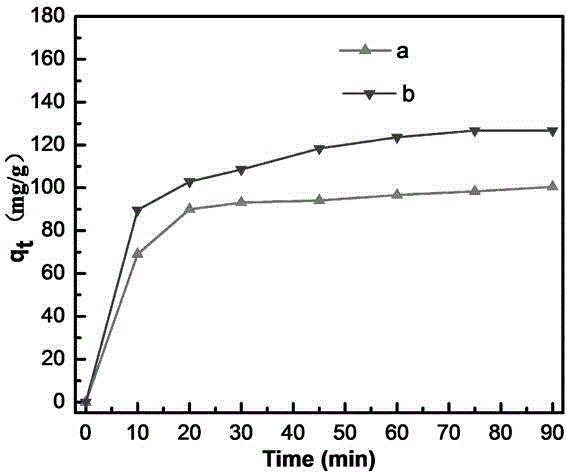
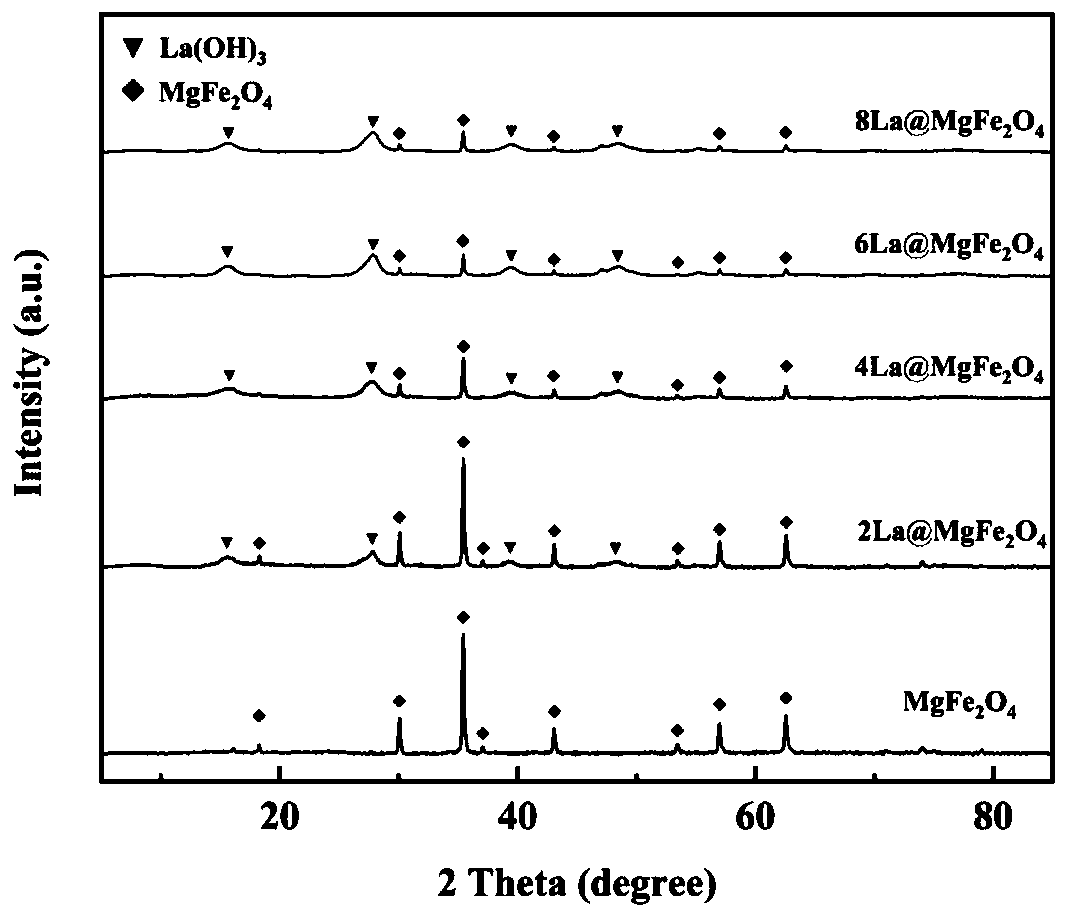
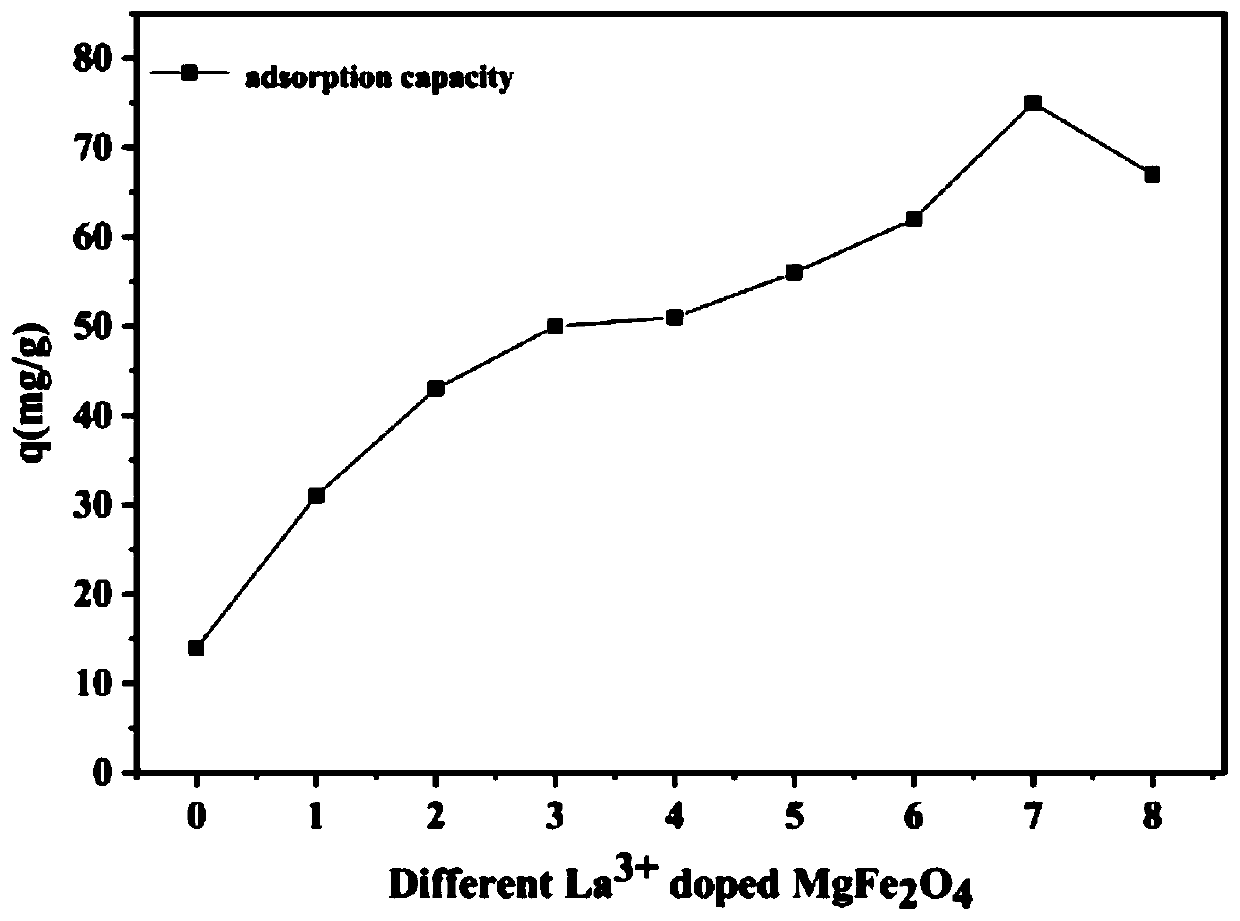
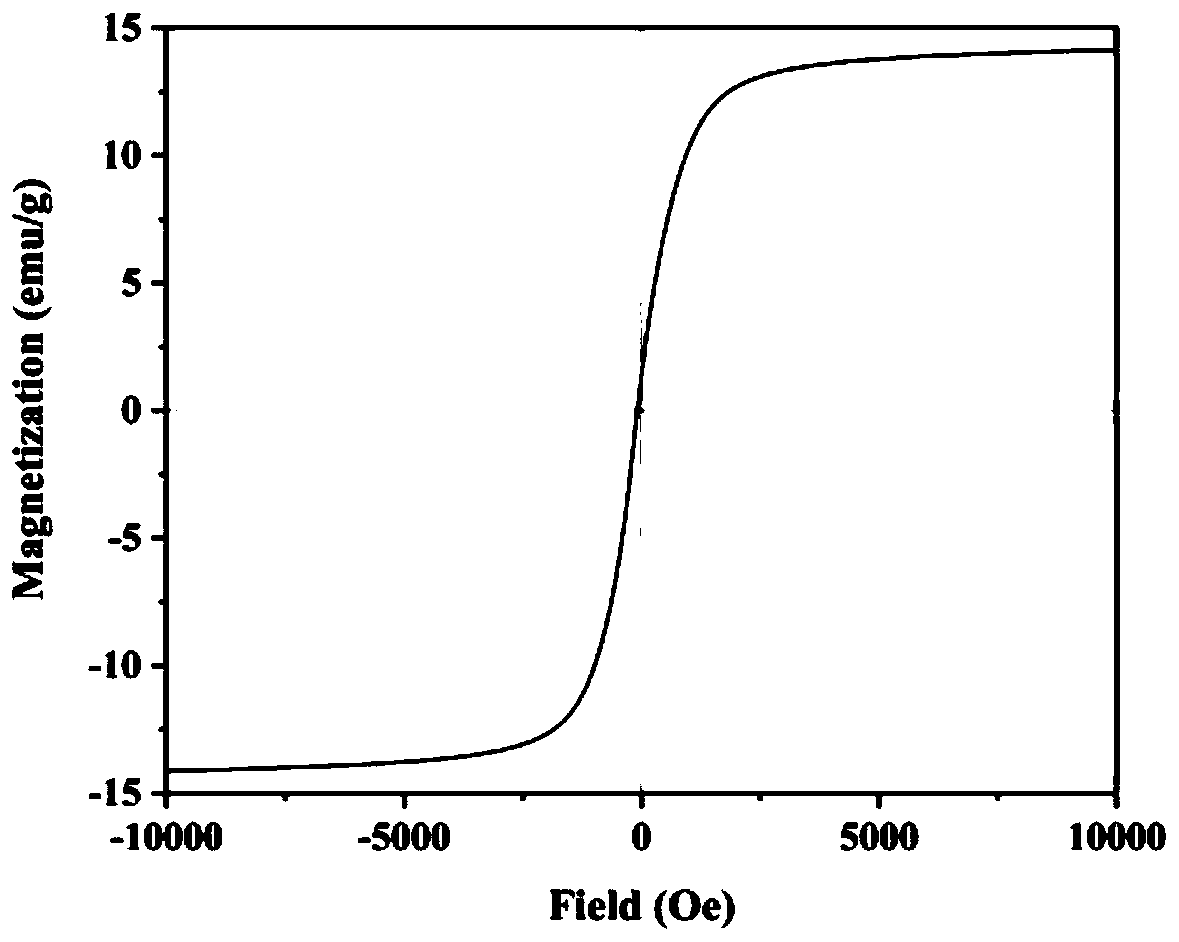
The invention discloses a lanthanum-doped magnesium ferrite composite material. A preparation method of the lanthanum-doped magnesium ferrite composite material comprises the following steps: (1) adding Fe3O4, magnesium chloride and lanthanum nitrate into water, and uniformly mixing to form a mixed solution, wherein the molar ratio of Fe3O4 to Mg<2+> to La<3+> is 2: 1: 1-8, preferably 2: 1: 7, and(2) adding a NaOH solution to enable the pH value of the solution to be greater than or equal to 10, reacting, and after the reaction is finished, separating out the product to obtain the lanthanum-doped magnesium ferrite composite material which is named as La@MgFe2O4. The invention discloses the lanthanum-doped magnesium ferrite composite material which can be used for adsorbing / removing phosphorus in sewage, has a high adsorption capacity for phosphate, is not influenced by coexisting anions in natural water, has a wide pH application range and a wide temperature application range (when the temperature is reduced to 10 DEG C, the adsorption capacity is almost the same as that at 25 DEG C), is slightly influenced by the temperature, can be recycled for multiple times, and can be separated by using a magnet.
Owner:INNER MONGOLIA UNIV OF TECH
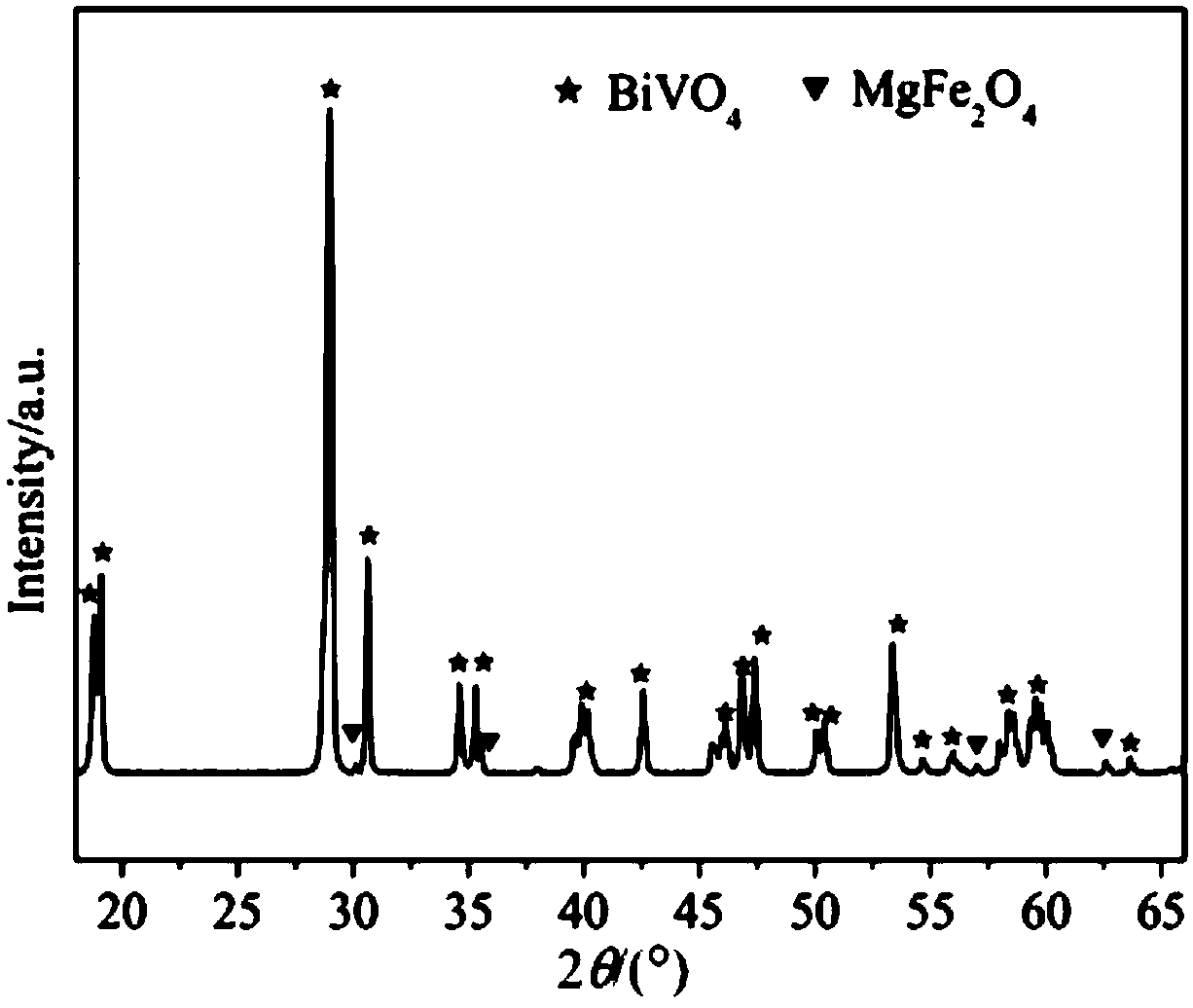
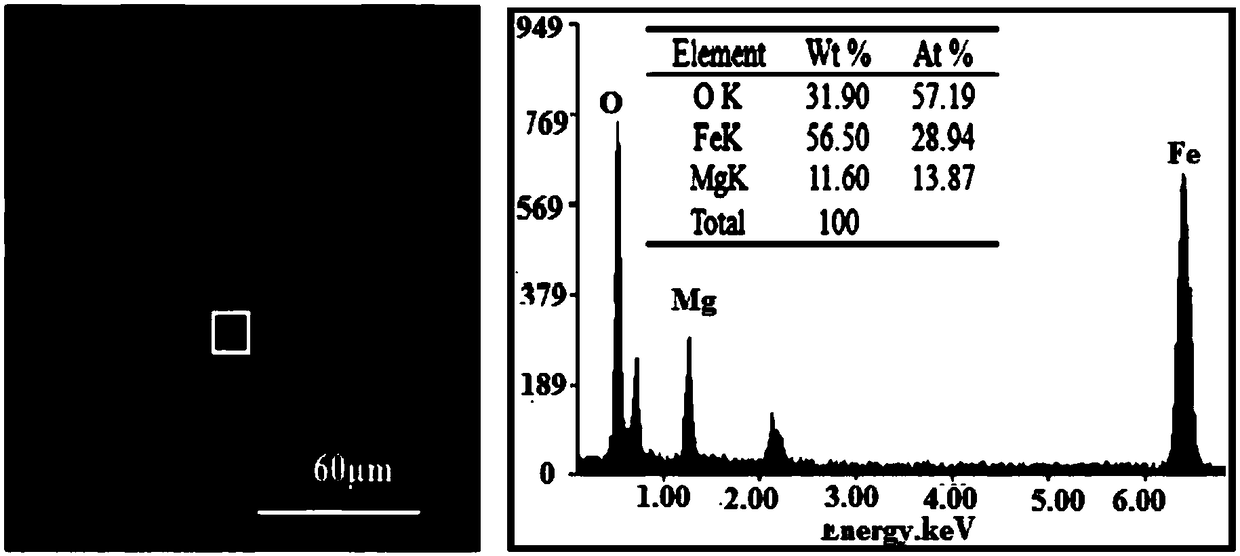
Bismuth vanadate-magnesium ferrite composite photocatalyst, and preparation method and application thereof
InactiveCN108636419AImprove performanceEasy to separate and recycleWater/sewage treatment by irradiationWater treatment compoundsHeterojunctionBismuth vanadate
The invention discloses a bismuth vanadate-magnesium ferrite composite photocatalyst, and a preparation method and application thereof. The photocatalyst comprises an m-BiVO4 substrate, wherein MgFe2O4 is dispersed on the surface of the m-BiVO4 substrate, and a mass ratio of the MgFe2O4 to the m-BiVO4 substrate is (5-20): (95-80). A monoclinic bismuth vanadate-magnesium ferrite composite photocatalysis material is prepared by using a low-temperature self-propagating sol-gel method and a calcination method; the photocatalytic properties of the prepared material are tested by using simulated visible light; and the superior photocatalytic properties of the material are proved by degradation of the non-biodegradable organic pollutant methylene orange. The material belongs to inorganic photocatalysis materials, and has high photocatalytic activity and good application prospects in environmental protection. The preparation method has the advantages of controllable morphology of monoclinic bismuth vanadate, good compounding between spinel-type magnesium ferrite and monoclinic bismuth vanadate, uniform dispersion and formation of effective p-n heterojunctions.
Owner:XI'AN UNIVERSITY OF ARCHITECTURE AND TECHNOLOGY
Mixtures of coated pigments and fatty acid salts for dyeing PVC
InactiveCN106459610AImprove thermal stabilityPigment treatment with non-polymer organic compoundsMagnesium saltInorganic compound
The invention relates mixtures of pigments containing at least one inorganic compound selected from among the group consisting of iron oxides, iron oxide hydroxides, zinc ferrites, zinc oxides, magnesium ferrites and manganese ferrites, said at least one inorganic compound being provided with a coating containing at least one magnesium or calcium hydroxide or oxide, and at least one calcium salt or magnesium salt of a fatty acid, methods for the production of said mixtures, the use thereof for dyeing polyvinyl chloride (PVC), methods for dyeing PVC, PVC dyed with mixtures of said type, and plastic products containing mixtures of said type.
Owner:LANXESS DEUTDCHLAND GMBH
Magnetic control frequency modulation far-infrared optical switch and implementation method thereof
InactiveCN106405972AChange transmittanceRealize magnetic field frequency modulation switchNon-linear opticsMagnetic susceptibilityDielectric substrate
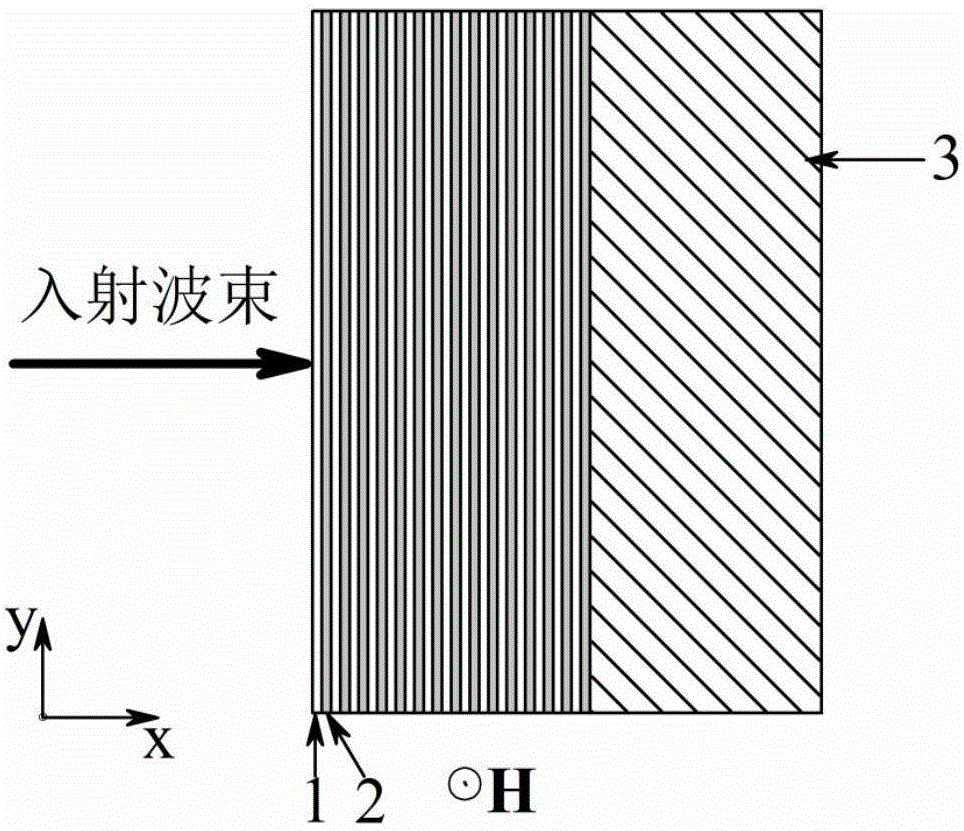
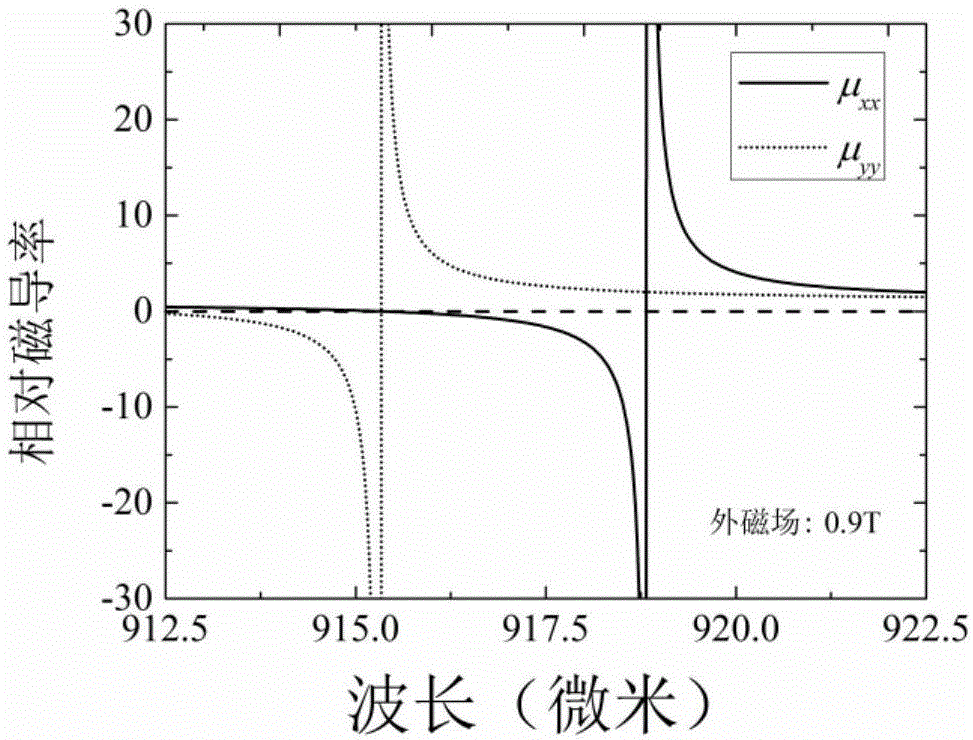
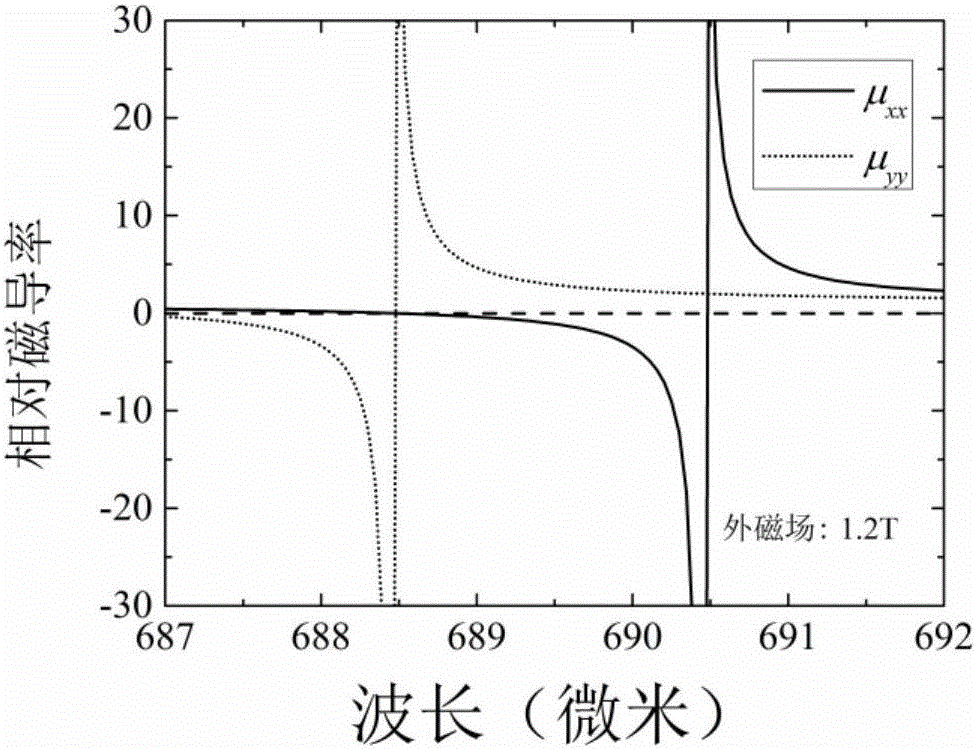
The invention relates to a magnetic control frequency modulation far-infrared optical switch which comprises a magnesium ferrite based metamaterial and a dielectric substrate which are arranged in sequence. The magnesium ferrite based metamaterial is formed by alternative overlapping of a magnesium ferrite film 1 and an aluminum oxide film 2 in the X-axis direction and is of a one-dimensional periodic layered structure. The thicknesses of the magnesium ferrite film 1 and the aluminum oxide film 2 in the X-axis direction are both smaller than one twentieth of the wave length of the selected electromagnetic wave for entering the optical switch. The lengths of the magnesium ferrite film 1 and the aluminum oxide film 2 in the Y-axis direction and Z-axis direction are both 10 times greater than the wave length. Accordingly, the magnesium ferrite based metamaterial can be obtained. The provided structure can control the magnetic susceptibility of the magnesium ferrite film under the regulation of an external magnetic field, the transformation of the magnesium ferrite based metamaterial from an ordinary non-magnetic medium to a magnetic hyperbolic medium is achieved, accordingly, the transmissivity of an incident TE polarized wave is controlled, and the optical switch is obtained.
Owner:TONGJI UNIV
Magnesium-aluminum refractory material
The invention relates to a refractory material, and particularly relates to a magnesium-aluminum refractory material. The magnesium-aluminum refractory material comprises the following mineral constituents: periclase (M), magnesium-alumina spinel (MA), forsterite (M2S), calcium-magnesium monticellite (CMS), and magnesium ferrite (MF). The magnesium-alumina spinel (MA) is high in melting point, small in thermal expansion, low in thermal stress, and good in thermal shock stability, has stable chemical property and strong resistance to basic slag, is a core for using magnesium-aluminum non-burning bricks, and is one of key materials for prolonging the service life. Therefore, the magnesium-aluminum refractory material has no seam melting loss and has better anti-slagging capacity and thermal shock resistance, the structure peeling phenomenon caused by the penetration of molten steel and slag can be overcome, and the service life is obviously prolonged; and as the product is non-burning or burnt at low temperature, energy resources can be saved, the cost can be lowered, the economic benefits are obvious, and thus the magnesium-aluminum refractory material is a high-quality refractory material.
Owner:QINGDAO YONGTONG ELEVATOR ENG
Method for synthesizing spinel structure magnesium ferrite nano particles
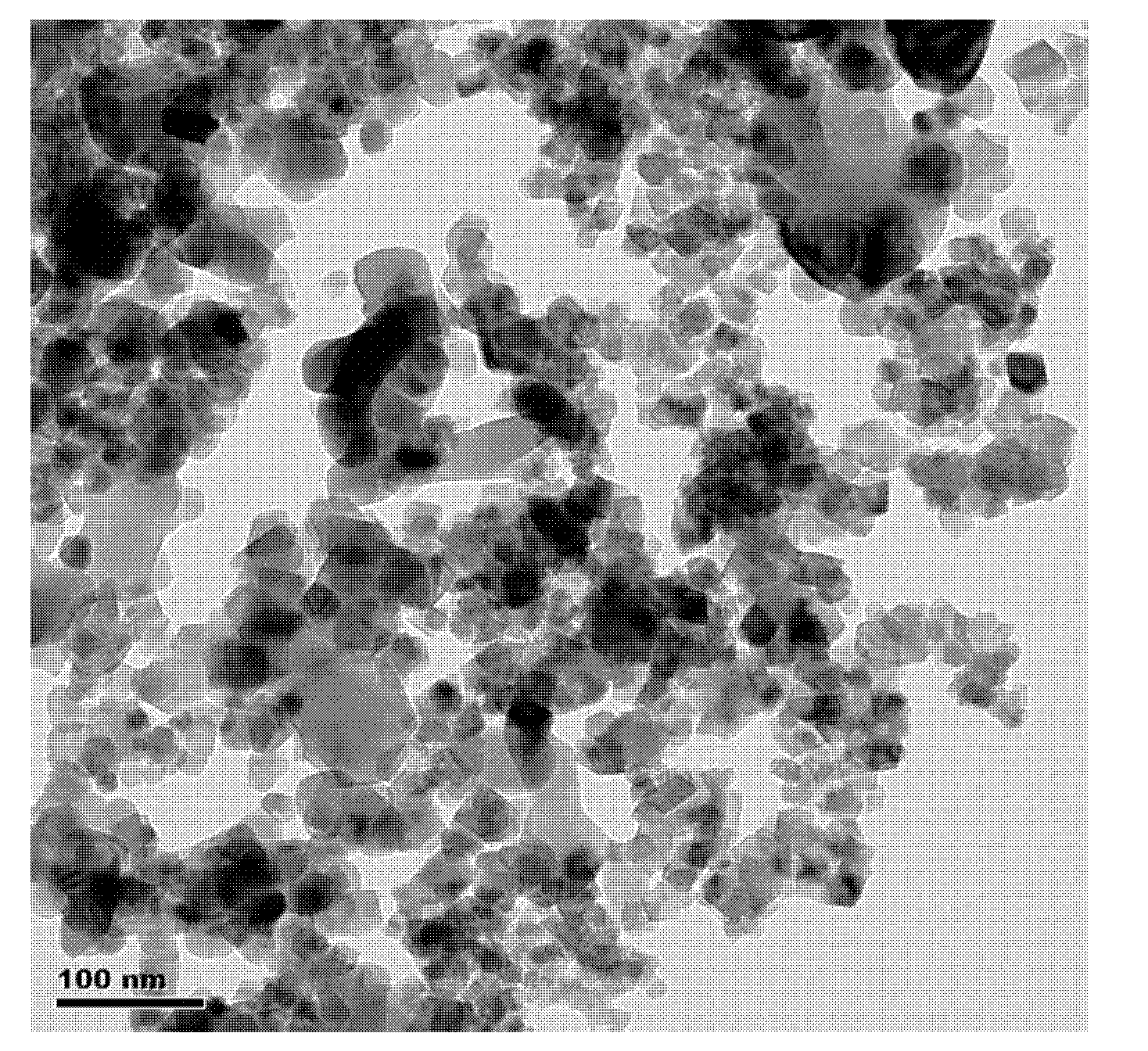
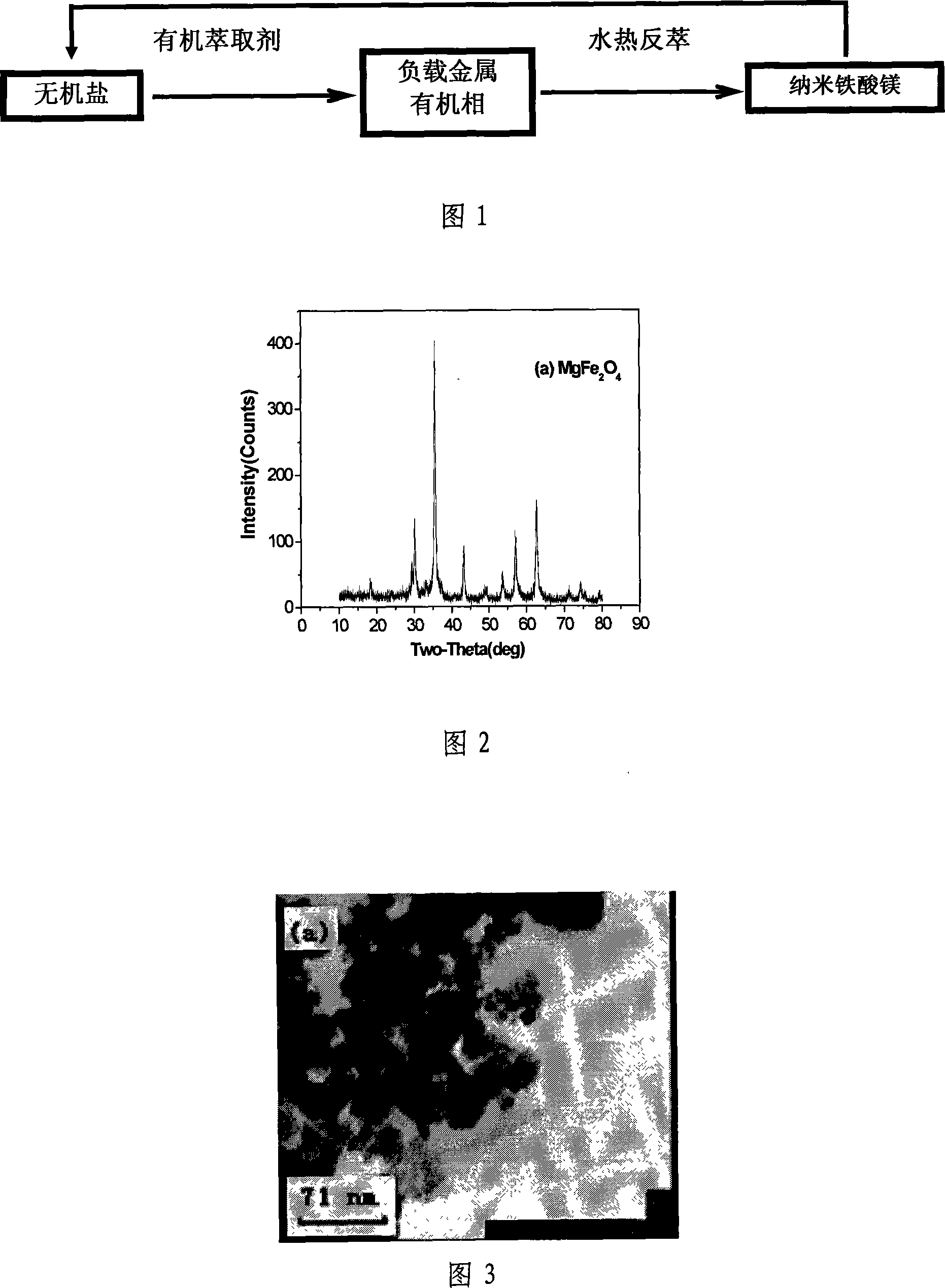
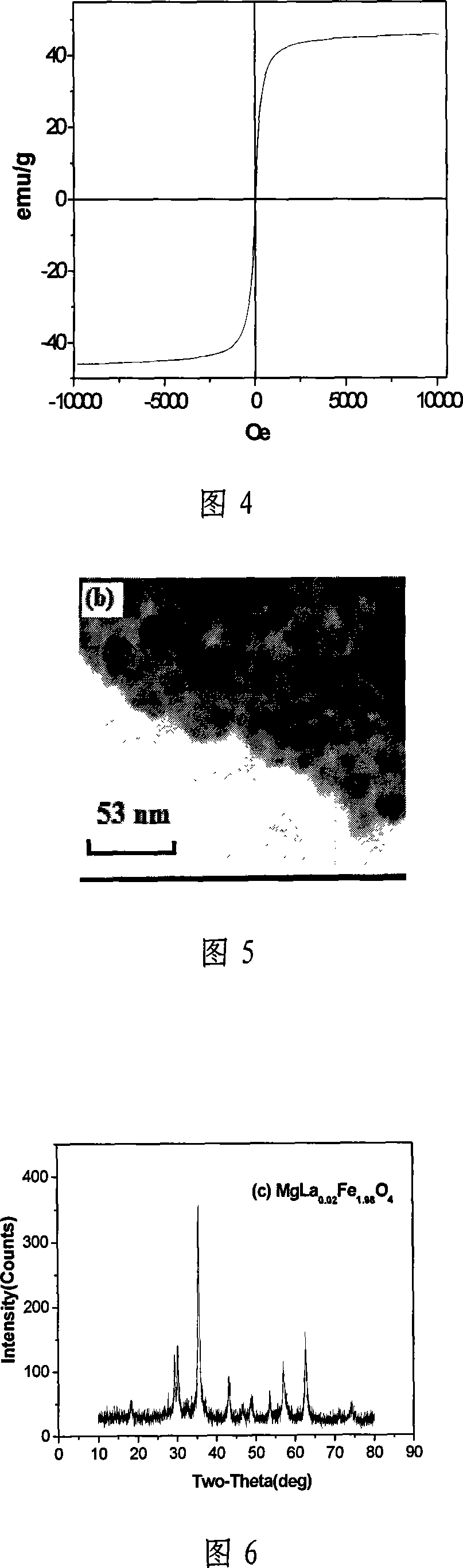
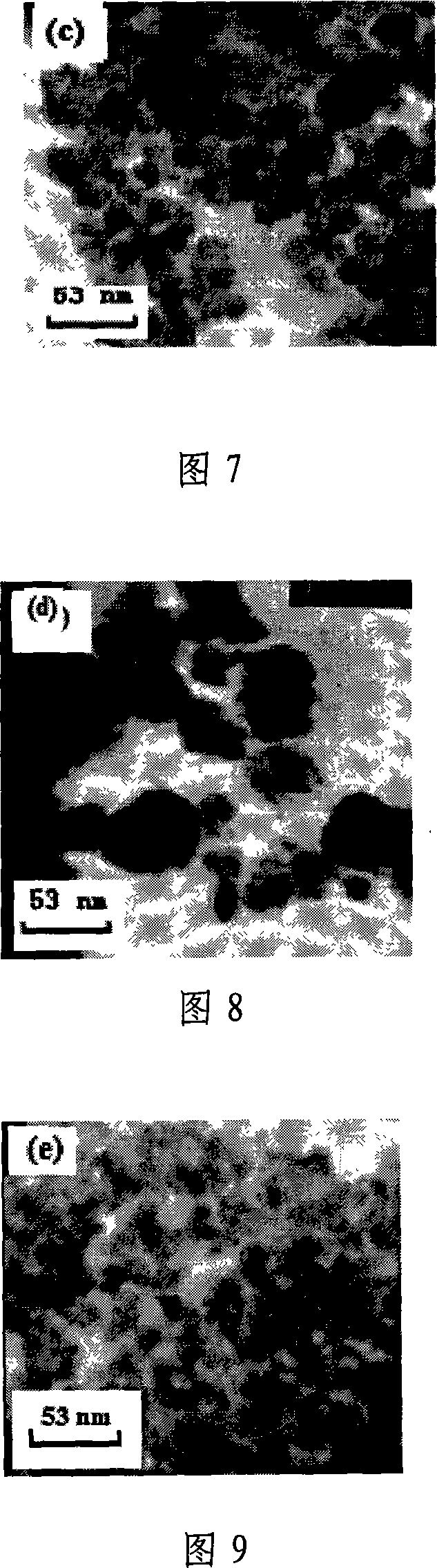
InactiveCN101070192BAvoid introducingReduce consumptionMagnesium compoundsIron compoundsAutoclaveMagnesium ferrite
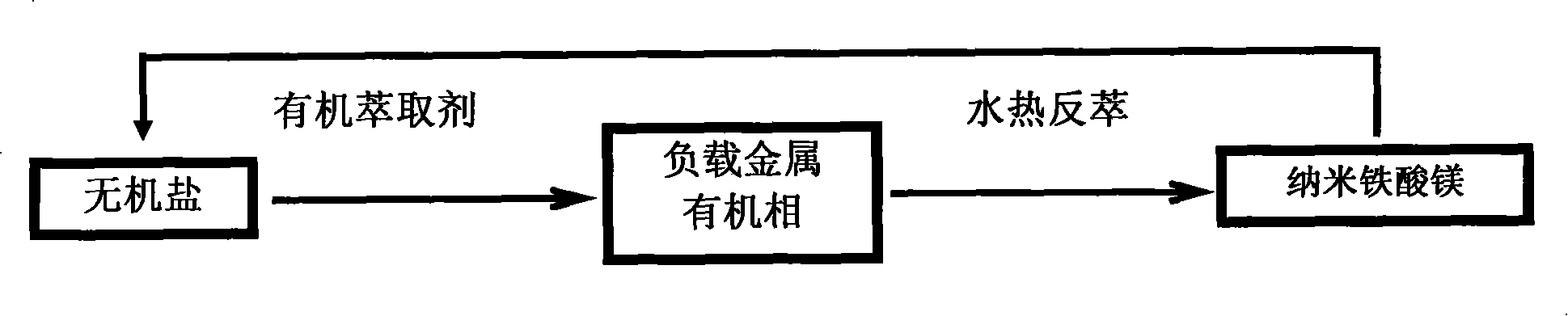
The invention relates to a kind of composed method of magnesium ferrite of spinel structure. It includes the following steps: extract trivalent iron ion and divalent iron ion in water phase through organic extractant, get predecessor of; absterge it with secondary distilled water for2-3 times and get pure load metal predecessor of organic phase; mix organic phase of Mg2+ and Fe3+ with mol rate of1:2 and introduce them into autoclave, add distilled water according to the rate of 3:1 or 4:1 of organic phase and water phase and airproof it, and then mix it fiercely, stripping with hydrothermal method at temperature between 200 degree C and 300 degree C and make them react for 2.5 hours; cool it to the room temperature under natural condition, separate liquid and centrifugalize it, absterge it with absolute alcohol for 2-3 times and dry it at temperature of 50 degree C for 30-180 minutes, then get product of magnesium ferrite. The organic material in the invention can be used circularly,the cost is low; the craft is simple and energy consumption id low; the discharge of the waste water is low; it is convenient to control the size of particle of oxide deposition in the range of supermicron.
Owner:TIANJIN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com