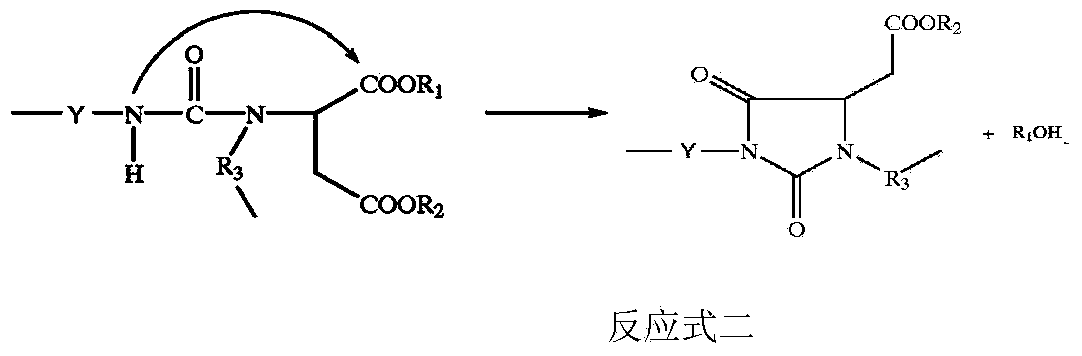
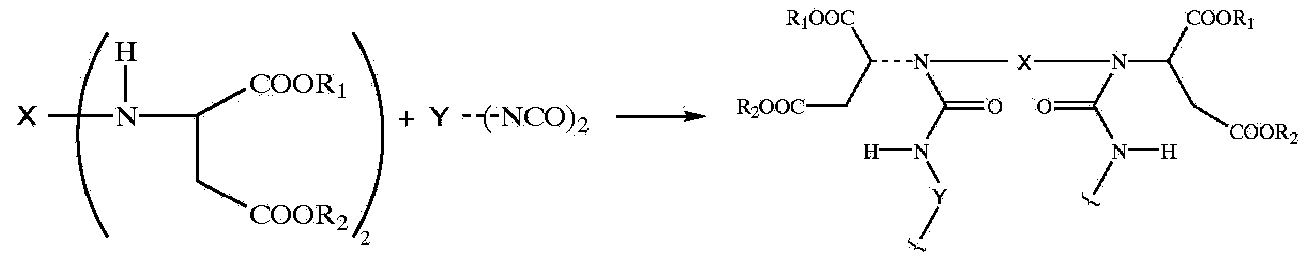Novel acylamino ester as well as synthesis method and application thereof
A primary amino group and alkyl group technology, which is applied in the preparation of carboxylic acid amides, chemical instruments and methods, and the preparation of organic compounds, etc., can solve the problems of unsuitable for industrial production, scarce sources of isomaleimide, and complicated synthesis methods And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0073] The synthetic 1 of example 1 unsaturated amide ester
[0074] In a 1000ml autoclave, 344 grams of diethyl maleate and 2.3 grams of sodium methylate catalyst were added, and then 108.2 grams of dimethylamine with a purity greater than or equal to 99% were introduced. Under stirring, slowly raise the temperature to 170°C. At this time, the pressure is 1.8-2.2Mpa. Keep the temperature and continue the reaction for 4 hours. After the pressure drops below 0.5Mpa., lower the temperature and pressure to end the reaction. Then the reactant was moved to a glass flask, and the remaining amine and alcohol were removed under vacuum, then neutralized with sodium hydroxide solution and washed with distilled water until the reactant was neutral. Finally, it is dehydrated by vacuum distillation, so that the moisture content is less than 0.1%, and the product obtained is N,N-dimethylmaleamide ethyl ester.
[0075]
example 2
[0076] The synthetic 2 of example 2 unsaturated amide esters
[0077]In a 1000ml autoclave, 288 grams of dimethyl maleate and 2.0 grams of sodium methoxide catalyst were added, and then 108.2 grams of dimethylamine with a purity greater than or equal to 99% were introduced. Under stirring, slowly raise the temperature to 170°C. At this time, the pressure is 1.8-2.2Mpa. Keep the temperature and continue the reaction for 4 hours. After the pressure drops below 0.5Mpa., lower the temperature and pressure to end the reaction. Then the reactant was moved to a glass flask, and the remaining amino alcohol was removed under vacuum, then neutralized with sodium hydroxide solution and washed with distilled water until the reactant was neutral. Finally, it is dehydrated by vacuum distillation, so that the moisture content is less than 0.1%, and the product obtained is N,N-dimethylmaleamide methyl ester.
[0078]
example 3
[0079] Synthesis of Example 3 Polyasparaginate 1
[0080] In the 1000ml glass three-necked flask that electric stirrer, thermometer and reflux condenser tube are housed, add the Jeffamine D230 (purchased from HUNTSMAN) methanol solution of 462 gram 50% concentration, under stirring, 342 gram example 1 synthesized N in half an hour , N-dimethylmaleamide ethyl ester was added dropwise into the reaction flask, and the temperature was kept at 70°C. The reaction was continued for 20 hours, and the polyasparagine product was obtained after removing methanol, which was designated as E3.
[0081]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com