Preparation method of bezoar ursodesoxycholic acid preparation
A technology of tauroursodeoxycholic acid and preparations, which is applied in anti-inflammatory agents, pill delivery, pharmaceutical formulations, etc. It can solve problems such as inability to produce, high requirements for capsule filling machines, and inability to maintain consistency, so as to ensure product quality quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Prescription 1 adopts the general and conventional dry granulation production process of pharmaceutical factories as follows: the process is simple to operate and has no special process parameter requirements.
[0033] (1), raw and auxiliary materials are all passed through 80 mesh sieves, for subsequent use;
[0034] (2), put tauroursodeoxycholic acid dihydrate, microcrystalline cellulose (PH101), lactose monohydrate, and corn starch in a multi-sport mixer for 20 minutes;
[0035] (3) Take the mixed material and put it on the dry granulator, adjust the rotation speed of the extrusion wheel (3.6~5.3rpm), the rotation speed of the feeding screw (12~24rpm), the pressure of the oil cylinder (the pressure of the oil cylinder is 1.0MPa~2.0MPa as the degree) , so that the three can effectively cooperate until the hardness of the pressed medicine block is moderate (the degree of being able to make particles with moderate hardness), and the material is collected;
[0036] (4),...
Embodiment 2~5
[0040] According to prescriptions 2-5, adopt the method described in Example 1 to prepare.
Embodiment 6
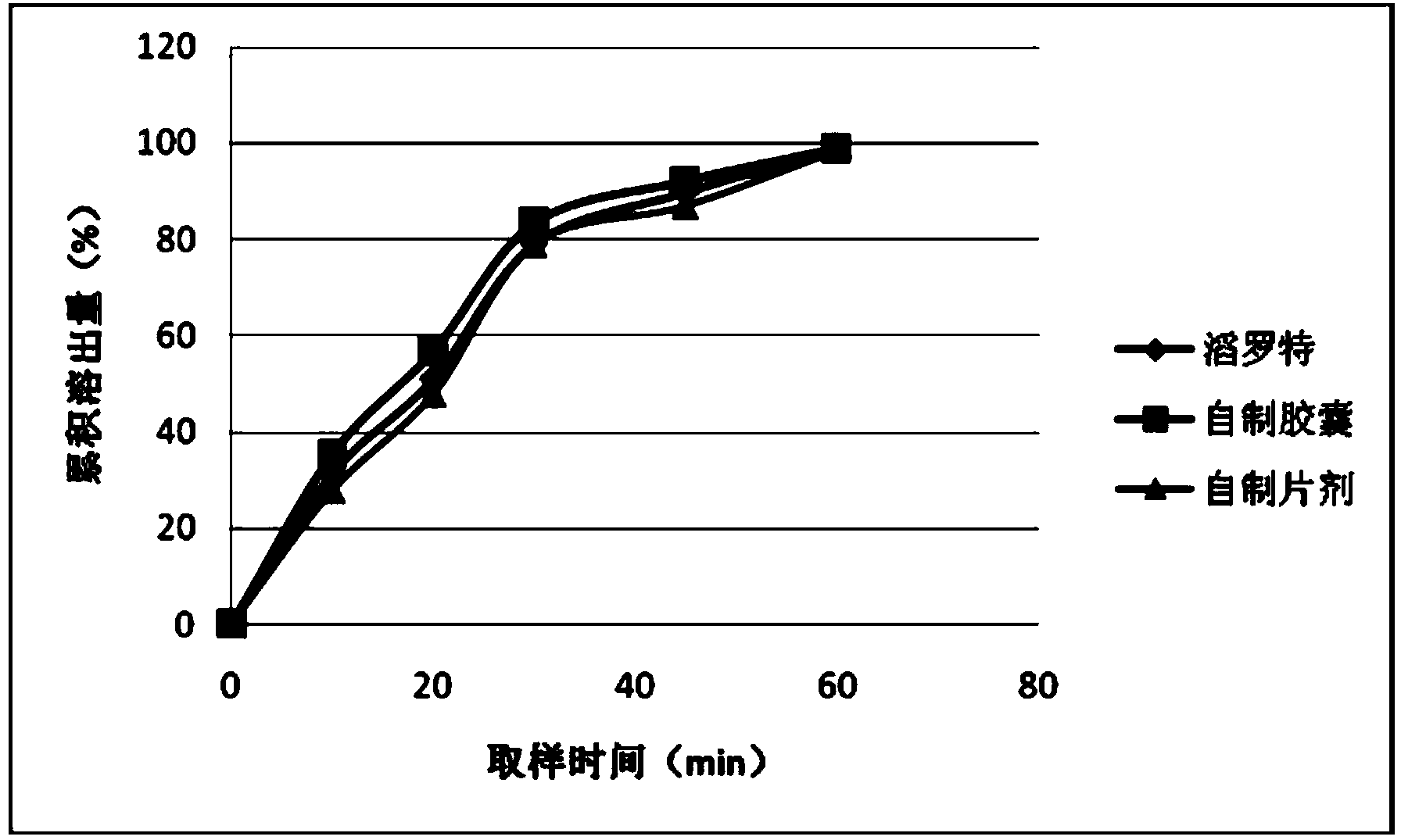
[0042] In vitro dissolution test method:
[0043]Paddle method, the dissolution medium is 900ml of aqueous solution, the rotation speed is 100 rpm, the temperature of the dissolution medium is 37°C ± 0.5°C, after 30 minutes, the solution is taken, filtered, and the determination of drug concentration is the HPLC method.
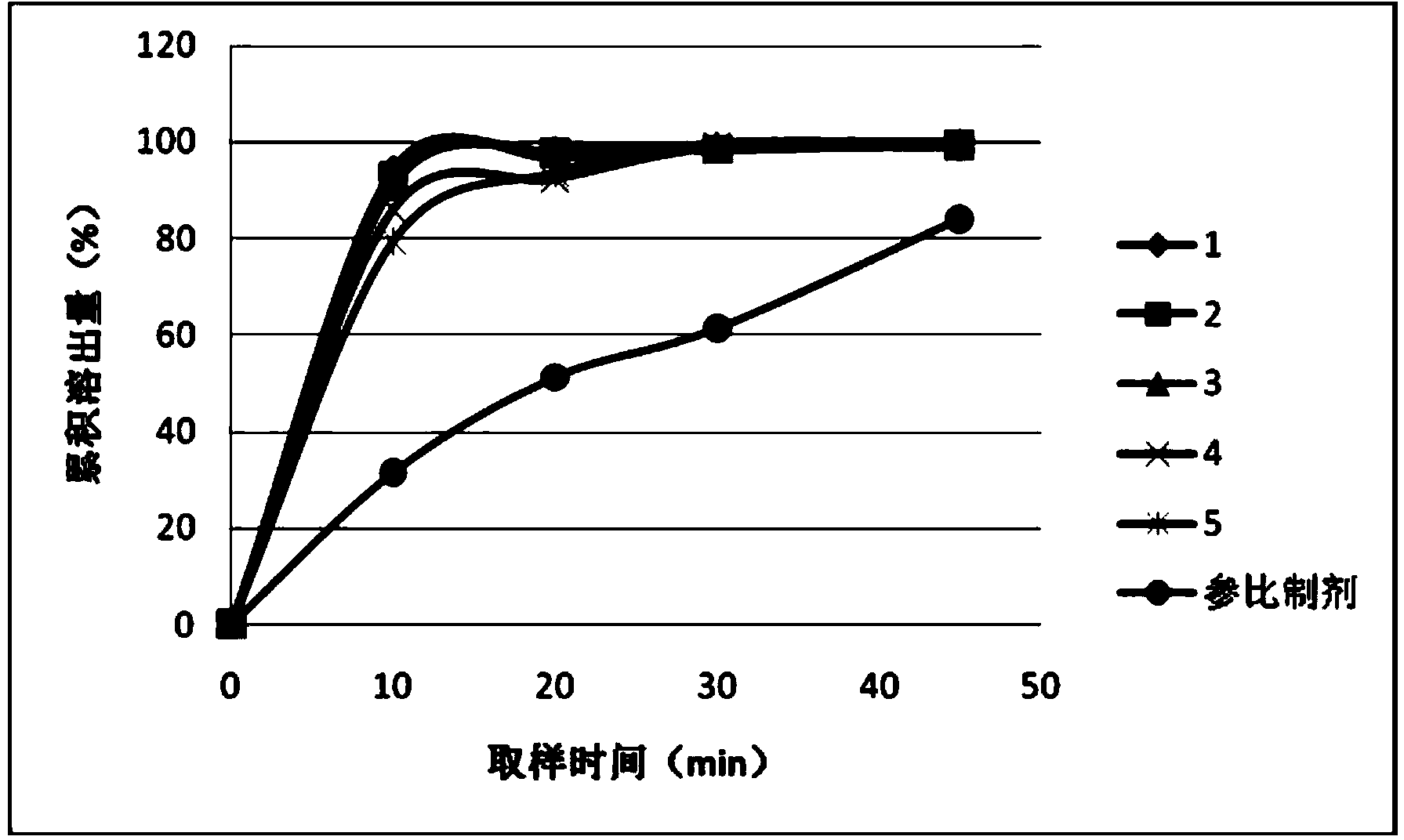
[0044] Determination data of dissolution curve of capsules for prescriptions 1 to 5:
[0045] prescription
10min
20min
30min
45min
1
94.4%
96.8%
99.0%
99.1%
2
93.1%
97.9%
98.4%
99.5%
3
90.8%
98.4%
99.1%
99.7%
4
86.1%
92.4%
98.9%
99.6%
5
79.4%
93.6%
99.7%
99.9%
31.4%
51.1%
61.3%
83.9%
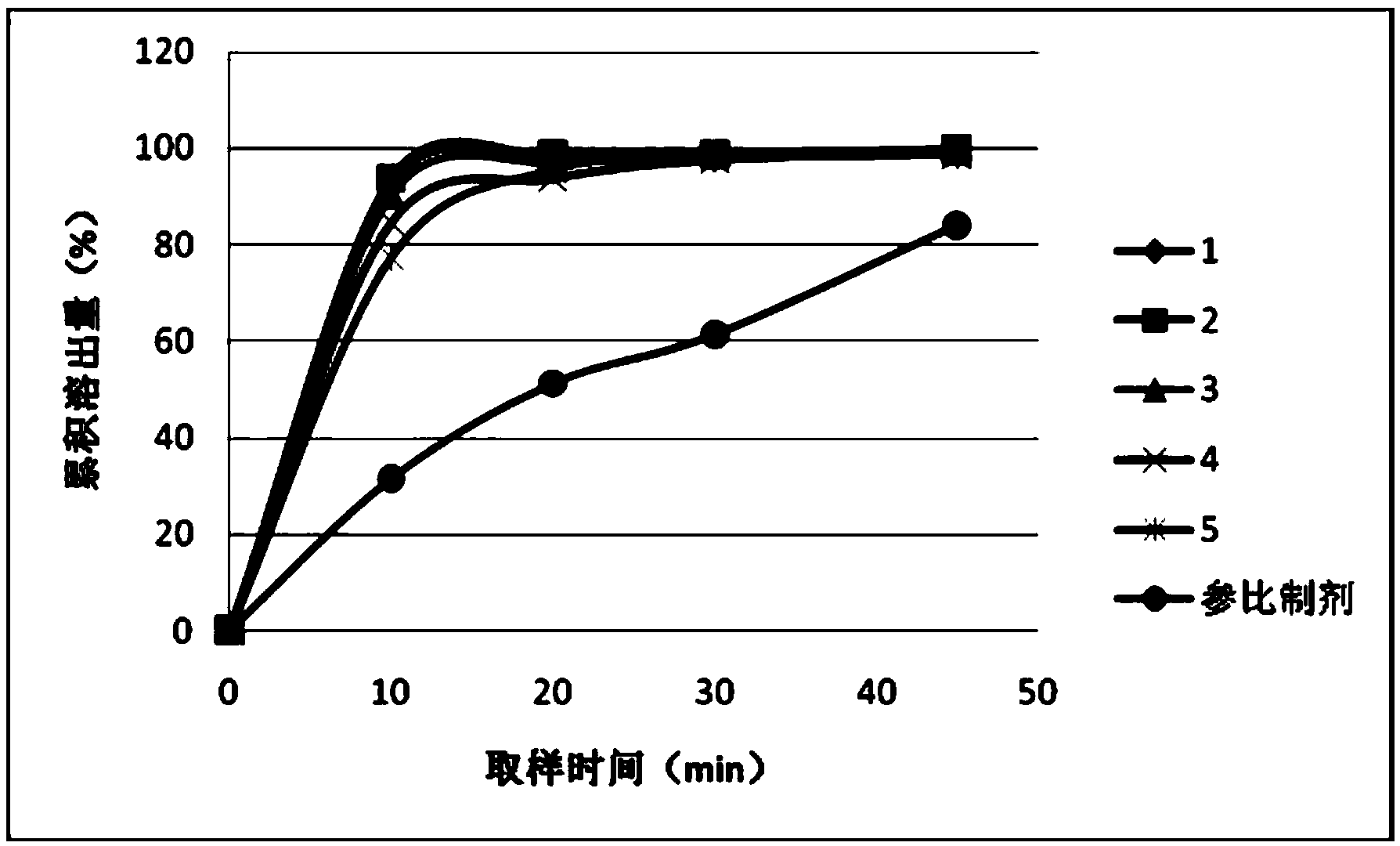
[0046] Determination data of dissolution profile of tablets from prescriptions 1 to 5:
[0047] prescription
10min
20min
30min
45min
1
92.8%
97.9% ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com