Method for recycling fluorine from phosphorus-containing fluorine slag and cogenerating calcium superphosphate
A calcium superphosphate, parallel technology, applied in the direction of fluorine/hydrogen fluoride, chemical instruments and methods, peroxide/peroxyhydrate/peroxyacid/superoxide/ozonide, etc., can solve expensive maintenance costs, reaction Problems such as high temperature and excessive sulfuric acid are used to achieve the effect of rapid and complete reaction, mild operating conditions and avoiding the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
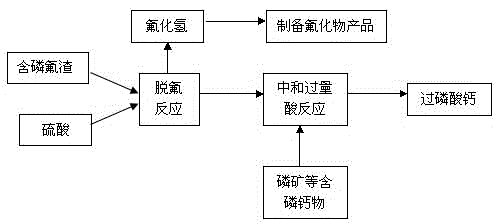
[0032] P will be calculated on a dry basis 2 o 5 1.7t of phosphorous and fluorine slag with 22% content, 24% F content and 70% water content and 950kg of 98% H 2 SO 4 Concentrated sulfuric acid was added to the defluorination reaction tank and stirred at 90°C for 25 minutes to react, that is, phosphorus-containing fluoride slag (calculated on a dry basis) and concentrated sulfuric acid (converted to pure H 2 SO 4 ) is added in an amount of 1:0.93, and the fluorine-containing gas released during the reaction is recovered by a fluorine absorption tower to generate about 1.1t of hydrofluoric acid (with a HF content of about 20%), which is then used to produce fluorides such as anhydrous hydrogen fluoride.
[0033] Phosphorus-containing fluoride slag and concentrated sulfuric acid were stirred and reacted in the defluorination reaction tank for 25 minutes, and P was added to the reaction slurry. 2 o 5 About 30% of phosphate rock powder 300kg stirring reaction for 4h to neutra...
Embodiment 2
[0035] P will be calculated on a dry basis 2 o 5 1.2t of phosphorous and fluorine slag with 28% content, 15% F content and 20% water content and 1t of 70% H 2 SO 4 Concentrated sulfuric acid was added to the defluorination reaction tank and stirred at 200°C for 40 minutes to react, that is, phosphorus-containing fluorine slag (calculated on a dry basis) and concentrated sulfuric acid (converted to pure H 2 SO 4 ) is added in an amount of 1:0.7, and the fluorine-containing gas released during the reaction is recovered by a fluorine absorption tower to generate about 0.94t of hydrofluoric acid (with a HF content of about 15%), which is then used to produce fluoride such as anhydrous hydrogen fluoride.
[0036] Phosphorus-containing fluoride slag and concentrated sulfuric acid were stirred and reacted in the defluorination reaction tank for 40 minutes, and P was added to the reaction slurry. 2 o 5 About 25% of phosphate rock powder 340kg stirring reaction for 4.5h to neutral...
Embodiment 3
[0038] P will be calculated on a dry basis2 o 5 2t of phosphorus and fluoride slag with 15% content, 30% F content and 100% water content and 1.1t 100%H 2 SO 4 Concentrated sulfuric acid was added to the defluorination reaction tank and stirred at 95°C for 3 hours, that is, phosphorus-containing fluorine slag (calculated on a dry basis) and concentrated sulfuric acid (converted to pure H 2 SO 4 ) is added in an amount of 1:1.1, and the fluorine-containing gas released during the reaction is recovered by a fluorine absorption tower to generate about 1.2t of hydrofluoric acid (with a HF content of about 25%), which is then used to produce fluorides such as anhydrous hydrogen fluoride.
[0039] Phosphorous and fluorine slag and concentrated sulfuric acid were stirred and reacted in the defluorination reaction tank for 3 hours, and P was added to the reaction slurry. 2 o 5 500kg of about 32% phosphate rock powder was stirred and reacted for 5 hours to neutralize the free acid ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com