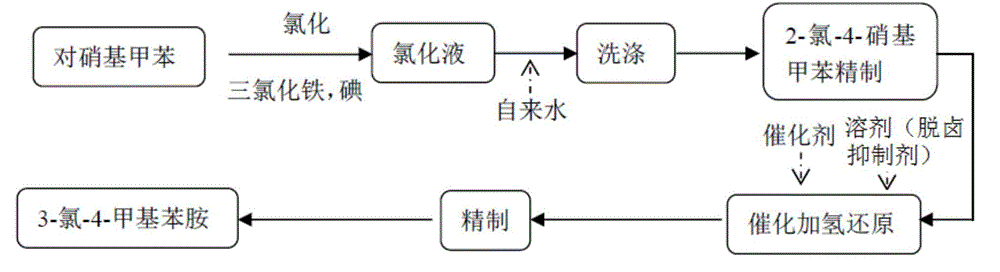
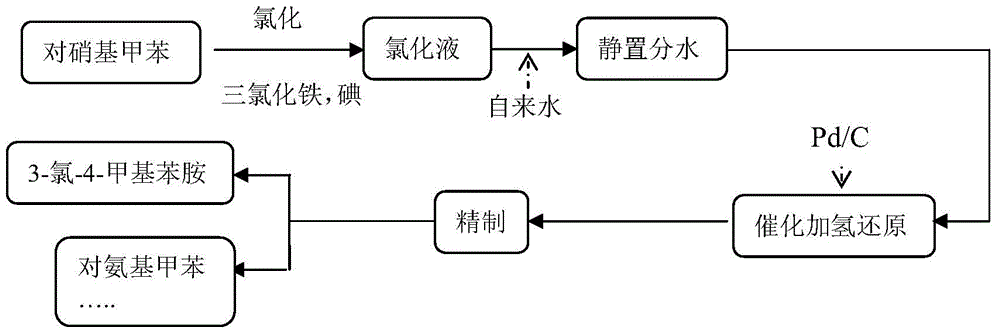
Method for synthesizing 3-chloro-4-methylaniline
A technology of methylaniline and dichloronitrotoluene, applied in chemical instruments and methods, preparation of organic compounds, preparation of amino compounds, etc., can solve problems affecting production efficiency, production capacity, reducing the effective use volume of reactors, and hydrogenation Catalyst poisoning deactivation and other problems, to achieve the effect of good inhibition of dechlorination performance, good lipophilicity and hydrophobicity, and strong resistance to poisoning
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
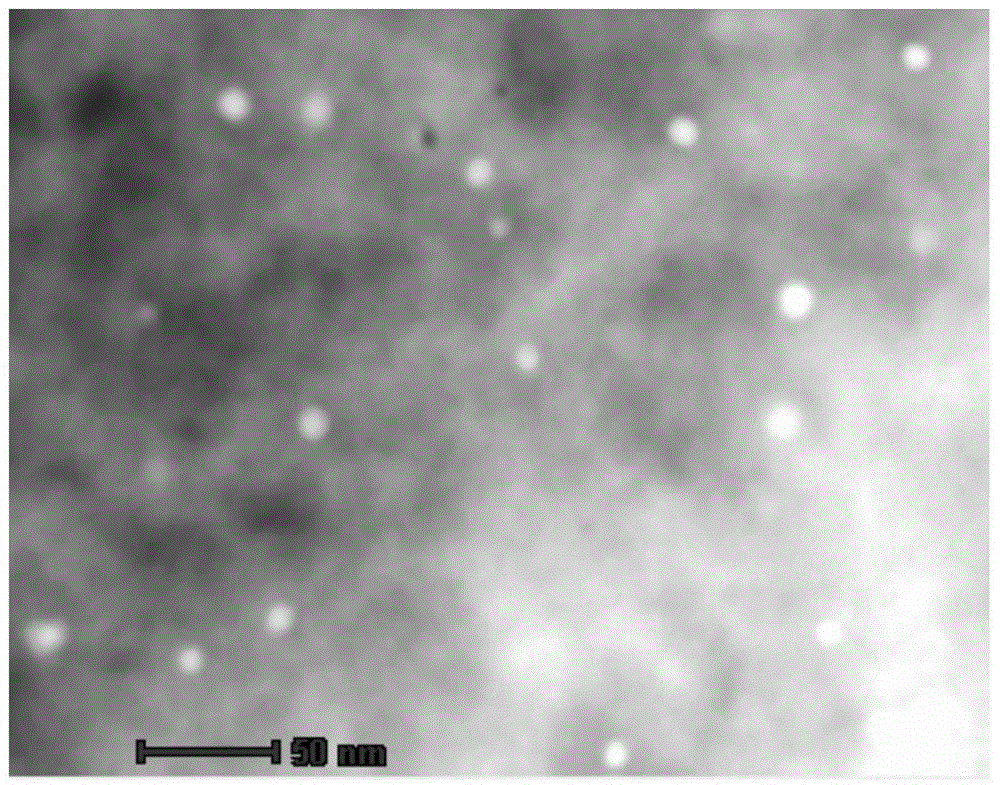
[0035] Weigh 10g of activated carbon, the specific surface area of activated carbon is 1500m 2 / g, heat treatment at 500°C for 5h under a hydrogen atmosphere. Then impregnate the activated carbon in 120 ml of 2.0 mol / l NaBr solution, stir at 45° C. for 4 hours, filter, recover the mother liquor for recycling, and wash the filter cake with deionized water until no Br ions are detected. Then the above-mentioned treated activated carbon was formulated into 100ml slurry at 80°C, and the corresponding H 2 PdCl 4 solution, stirred for 5h, with 10% Na 2 CO 3 Solution Adjust the pH of the solution to 7.0, and then control the temperature at 30°C. Stir for 0.5h, filter, and wash with deionized water until no chloride ions are detected. Then reduce at 200° C. for 2 h under a hydrogen atmosphere to obtain the carbon-supported palladium catalyst. Statistical analysis of 500 random palladium particles by a high-power transmission electron microscope shows that 85% of the particle s...
Embodiment 2
[0037] Weigh 10g of activated carbon, the specific surface area of activated carbon is 1200m 2 / g, heat treatment at 400°C for 5h under a hydrogen atmosphere. The activated carbon was then immersed in 120 ml of 2.5 mol / l NaBr solution, stirred at 45°C for 4 hours, filtered, the mother liquor was recovered and recycled, and the filter cake was washed with deionized water until no Br ions were detected. Then the above-mentioned treated activated carbon was formulated into 100ml slurry at 80°C, and the corresponding H 2 PdCl 4 solution, stirred for 6h, with 15% Na 2 CO 3 Solution The pH of the solution was adjusted to 7.0, followed by temperature control at 35°C. Stir for 0.5h, filter, and wash with deionized water until no chloride ions are detected. Then reduce at 200° C. for 2 h under a hydrogen atmosphere to obtain the carbon-supported palladium catalyst. Statistical analysis of 500 random palladium particles by a high-power transmission electron microscope shows that...
Embodiment 3
[0039] Weigh 10g of activated carbon, the specific surface area of activated carbon is 1500m 2 / g, heat treatment at 350°C for 5h under a hydrogen atmosphere. Then this gac is immersed in the KBr solution of the 2.5mol / l concentration of 120ml, 45 DEG C of constant temperature stirring 5h, filter, mother liquor recycles mechanically, and filter cake is washed with deionized water until no Br ion is detected. Then the above-mentioned treated activated carbon was formulated into 100ml slurry at 80°C, and the corresponding H 2 PdCl 4 solution, stirred for 4h, with 10% Na 2 CO 3 Solution The pH of the solution was adjusted to 7.0, followed by temperature control at 35°C. Stir for 1 h, filter, and wash with deionized water until no chloride ions are detected. Then reduce at 250° C. for 2 h under hydrogen atmosphere to obtain the carbon-supported palladium catalyst. Statistical analysis of 500 random palladium particles by a high-power transmission electron microscope shows ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com