Method for preparing human immunoglobulin for intravenous injection
A technology of human immunoglobulin and intravenous injection, which is applied in the field of preparation of intravenous human immunoglobulin, can solve problems such as easy occurrence of adverse reactions, and achieve the effects of good market application prospects, high biological activity and low content of impurity factors.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
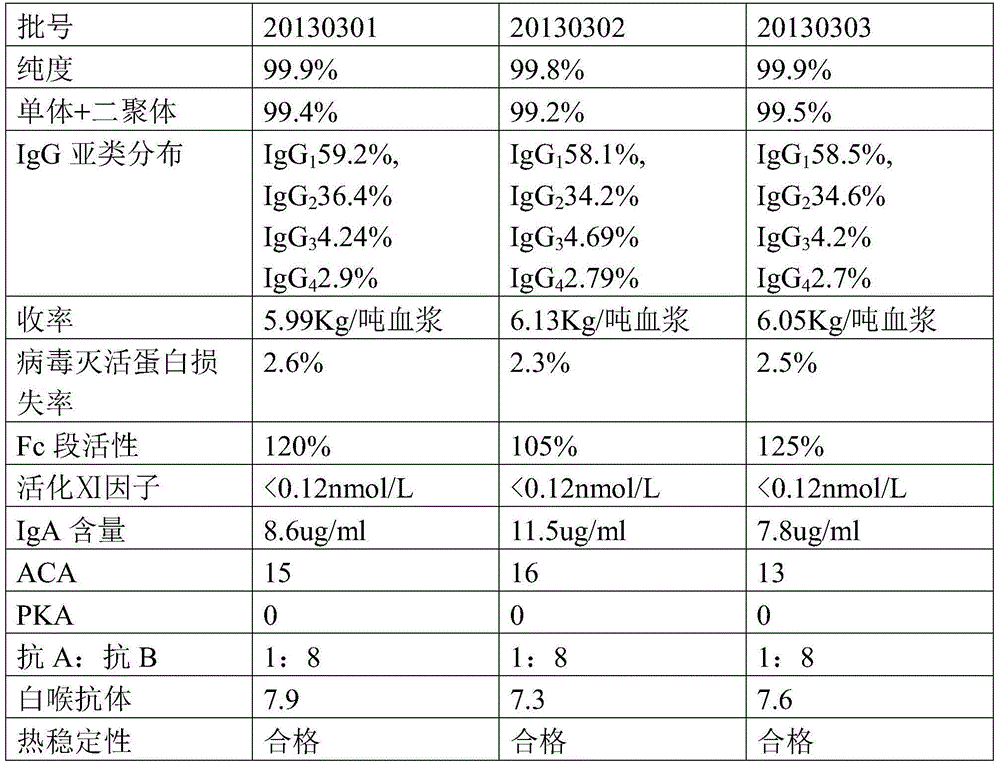
Embodiment 1
[0038] Embodiment 1 uses the present invention to prepare the production method of intravenous injection human immunoglobulin
[0039] 1. Experimental materials
[0040] The Cohn method component I+II+II precipitation (FI+II+II) was prepared according to the low-temperature ethanol precipitation method recorded in "Medical Biological Products" (People's Health Publishing House), second edition, page 1194.
[0041] 2. Experimental method
[0042] In the following methods, in the steps involving stirring, the stirring frequency is 70-90 rpm / min, and the purpose of stirring is to fully mix the solution.
[0043] 2.1 Confirmation of Cohn method component Ⅰ+Ⅱ+Ⅱ precipitation (F Ⅰ+Ⅱ+Ⅱ precipitation)
[0044] 2.1.1 The original production plasma used for FⅠ+Ⅱ+Ⅱ precipitation should comply with the provisions of the "Chinese Pharmacopoeia" on the regulation of human plasma used in the production of blood products.
[0045] 2.1.2 Before starting the operation, confirm the batch numb...
experiment example 1
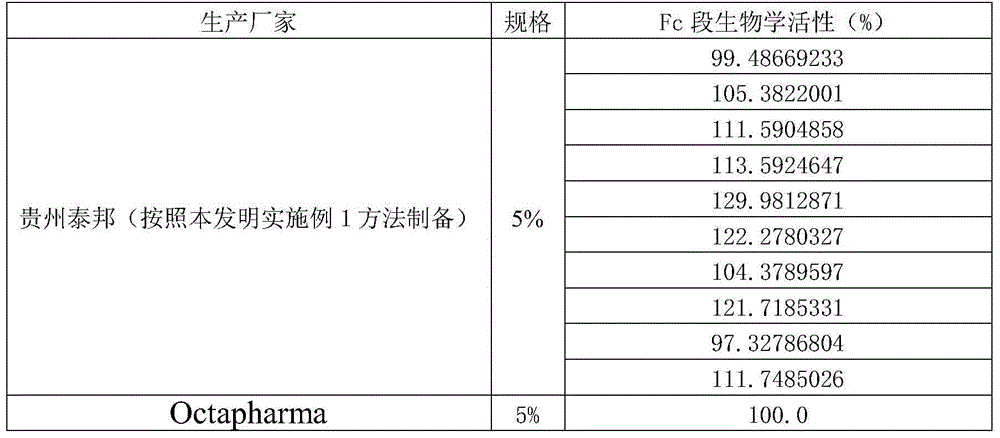
[0139] Experimental Example 1 Detection of the Fc segment of the intravenous injection of human immunoglobulin products of the present invention
[0140] 1. Experimental materials
[0141] 1.1 Medicine The present invention injects human immunoglobulin (5% 2.5g / bottle, batch number: 20130511, 20130512, 20130513, 20130514, 20130515, 20130516, 20130617, 20130618, 20130619), Octapharma Biological Products Co., Ltd. produces intravenous human immunoglobulin Protein 5%, batch number: C141E8532), intravenous injection of human immunoglobulin reference standard (for Fc segment function and molecular weight detection): European Medicines and Medical Quality Administration, batch number Y0001512.
[0142] 2. Detection method
[0143] Prepare the reference standard solution:
[0144] a. European reference standard solution
[0145] Adjust the pH of the reference product to 6.8-7.0 with sodium hydroxide solution, and then dilute the IgG concentration of the reference product to 40 mg / ...
experiment example 2
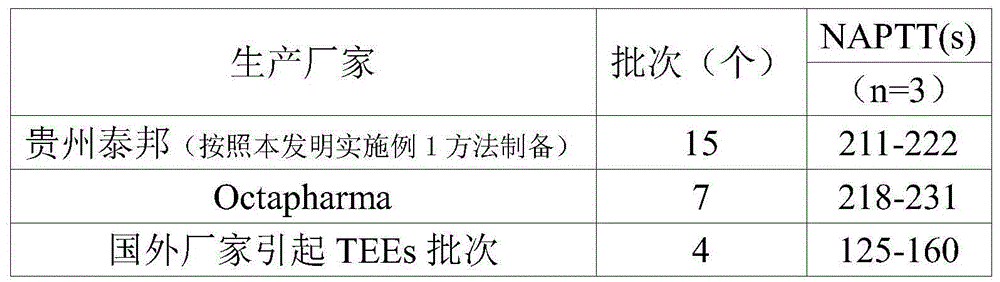
[0156] Experimental example 2 Detection of blood coagulation activator level of intravenous injection of human immunoglobulin products of the present invention
[0157] 1. Experimental materials
[0158] 1.1药品本发明静注人免疫球蛋白(批号:2090101、20090102、20090203、20100202、20100916、20101004、20100921、20101221、20101209、20101221、20110615、20110716、20110819、20110614、20110820),Octapharma生物制品有限公司生产 Intravenous injection of human immunoglobulin (5%, lot number: A125A853C, A140B8541, C141E8532; 10%, lot number: A105C843J, A127A8439C148A843B, B148A8444)
[0159] 1.2 Main reagents and consumables
[0160] Blood coagulation factor Ⅱ, Ⅴ, Ⅶ, Ⅸ, Ⅹ, Ⅺ, Ⅻ deficient plasma: American Pacific Company; APTT reagent: Chengdu Concord Biotechnology Center; PT reagent: Chengdu Concord Biotechnology Center; corn trypsin inhibitor (CTI): Germany Merck Calbiochem; FⅪa standard: Merck Calbiochem, Germany; thrombin fluorescent peptide substrate: Bachem, Switzerland; cephalin: American Roche diagnostics;
[0161] 1.3 Ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com