Anticoagulant compound, preparation method and application of anticoagulant compound and drug composition containing anticoagulant compound
A compound, drug technology, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
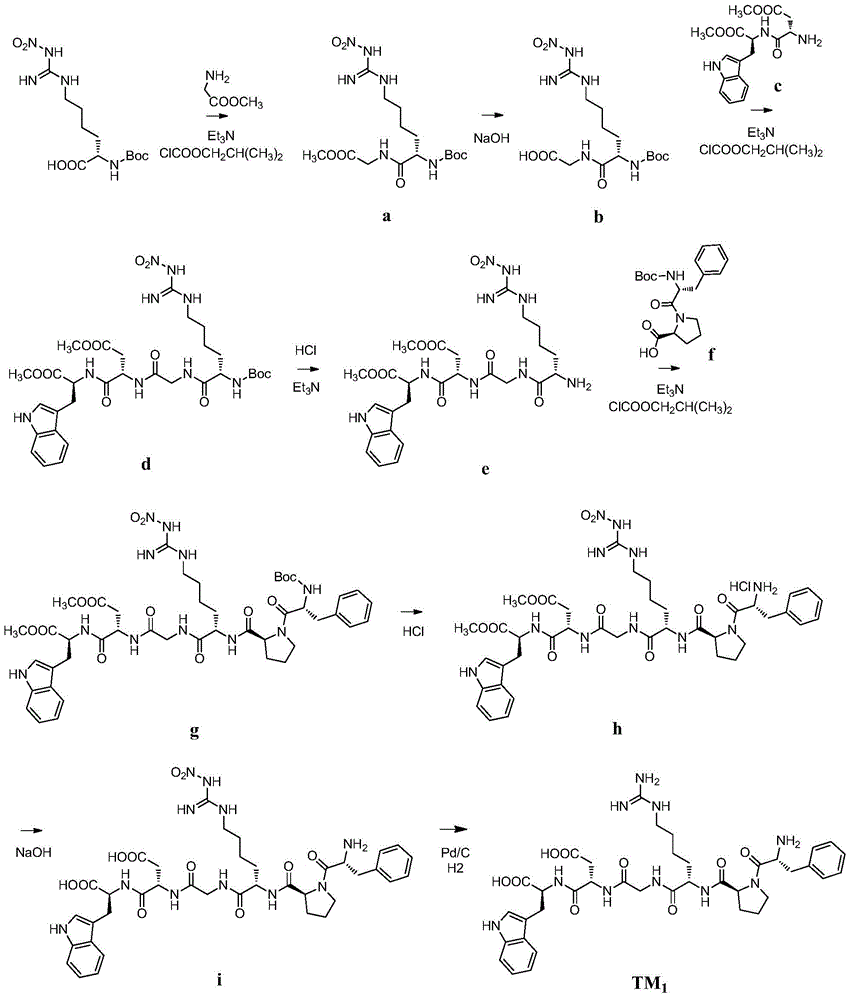
[0086] Example 1TM 1 preparation of
[0087]
[0088] 1) Glycine methyl ester hydrochloride (37.7g, 0.3mol) was dissolved in an appropriate amount of dichloromethane, and 41.68ml of triethylamine was added under stirring conditions to produce a large number of flocs, which were filtered off, and the filtrate was concentrated to obtain a white solid Glycine methyl ester, without treatment, directly reacted.
[0089] Add 450mL dry tetrahydrofuran to the reaction flask, add N G -Nitro-N 2 -Boc-L-homoarginine (33.3g, 0.1mol, purchased from Jill Biochemical (Shanghai) Co., Ltd.), cooling the reaction solution to -20°C, adding triethylamine (10.1g, 0.1mol) in turn under stirring and isobutyl chloroformate (13.6 g, 0.1 mol). After 30 minutes, glycine methyl ester was added to the reaction solution, stirred at -20° C. for 30 minutes, and then the temperature of the reaction solution was returned to room temperature. After the reaction solution was concentrated under reduced pr...
Embodiment 2
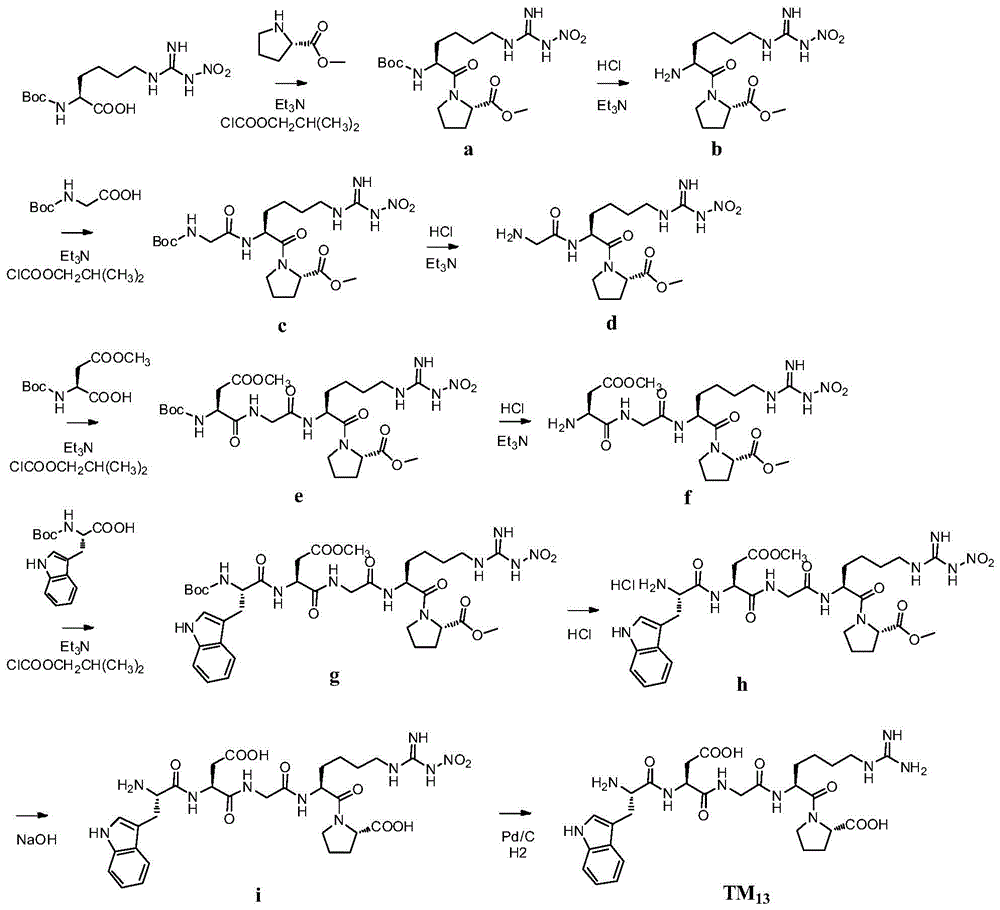
[0104] Example 2TM 13 preparation of
[0105]
[0106] 1) Dissolve L-proline methyl ester hydrochloride (49.5g, 0.3mol) in dichloromethane, add 41.68ml triethylamine under stirring condition, white flocs are formed, filter off the precipitate, and reduce the filtrate to Concentrate under reduced pressure to obtain white solid L-proline methyl ester, which is directly used for the next reaction without further treatment.
[0107] Add 300 mL of dry tetrahydrofuran to the reaction flask, add N G -Nitro-N 2 -Boc-L-homoarginine (33.3g, 0.1mol), cooling the reaction solution to -20°C, adding triethylamine (10.1g, 0.1mol) and isobutyl chloroformate (13.6g, 0.1mol). After 30 minutes, L-proline methyl ester was added to the reaction solution, stirred at -20° C. for 30 minutes, and then the temperature of the reaction solution was returned to room temperature. After the reaction solution was concentrated under reduced pressure, 300 mL of ethyl acetate was added, and after dissol...
experiment example 1
[0118] In vitro inhibitory effect of experimental example 1 on human thrombin
[0119] Measuring principle:
[0120] The p-nitroaniline obtained by thrombin-catalyzed hydrolysis of the substrate S-2238 has an absorption at 405nm. Therefore, the absorption value of p-nitroaniline at 405 nm can directly reflect the strength of thrombin activity, and the effect of the compound on thrombin activity can be observed in vitro.
[0121] test methods:
[0122] Add 8 μM substrate S-2238 (H-D-Phe-Pip-Arg-p-nitroaniline·2HCl) and a specific concentration of compound to 1 mL of phosphate buffer containing 0.1% PEG and 1% DMSO, pH 7.2. Add 10 mU of thrombin extracted from human plasma. After 10 minutes, absorbance was measured at 405 nm.
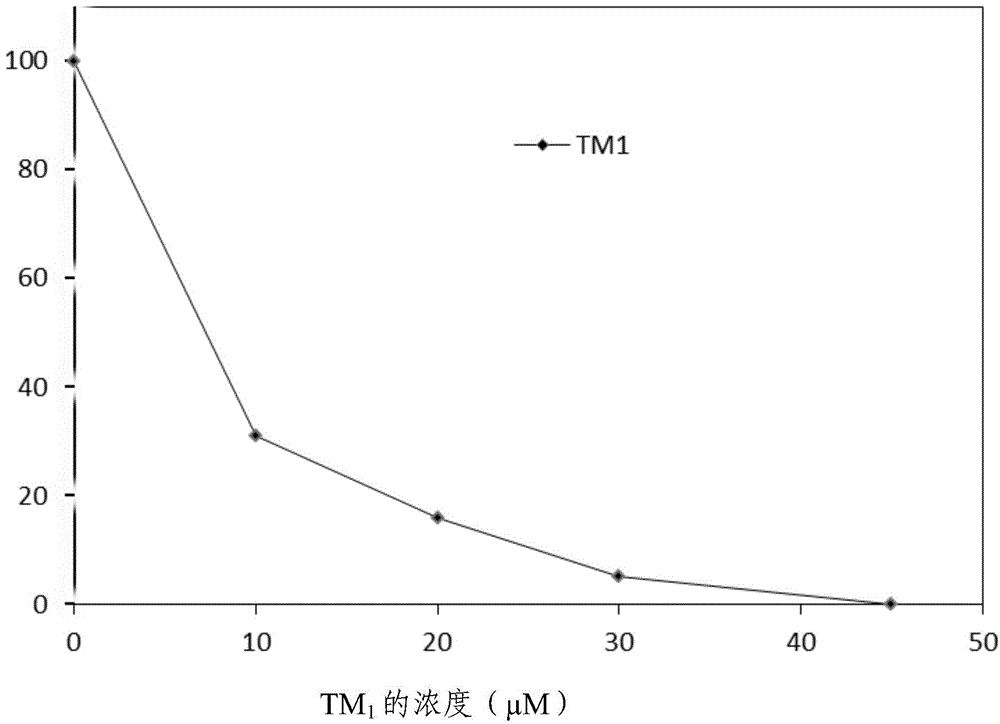
[0123] Results: TM was determined separately 1 -TM 20 and argatroban at a final concentration of 10 -4 M, 10 -5 Inhibition rate of human thrombin at M; measure and calculate the half inhibitory concentration IC of the compound on thrombin 50 . T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com