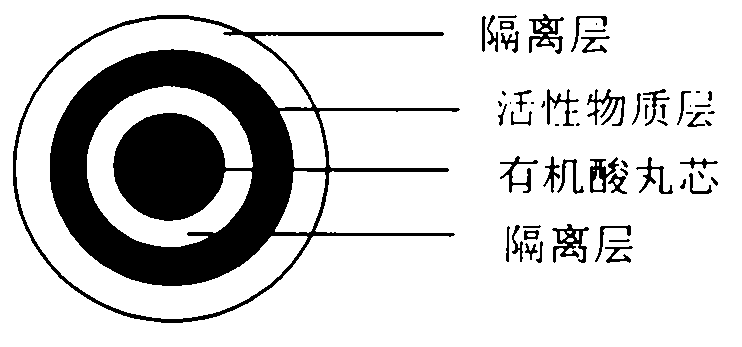
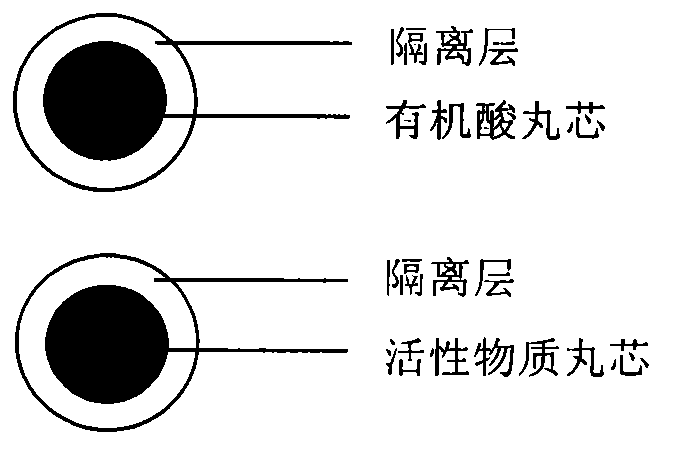
Pellet medicine composition containing pradaxa or salt and hydrate thereof
A technology of composition and hydrate, applied in the field of medicine, can solve problems such as difficult control and complicated process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
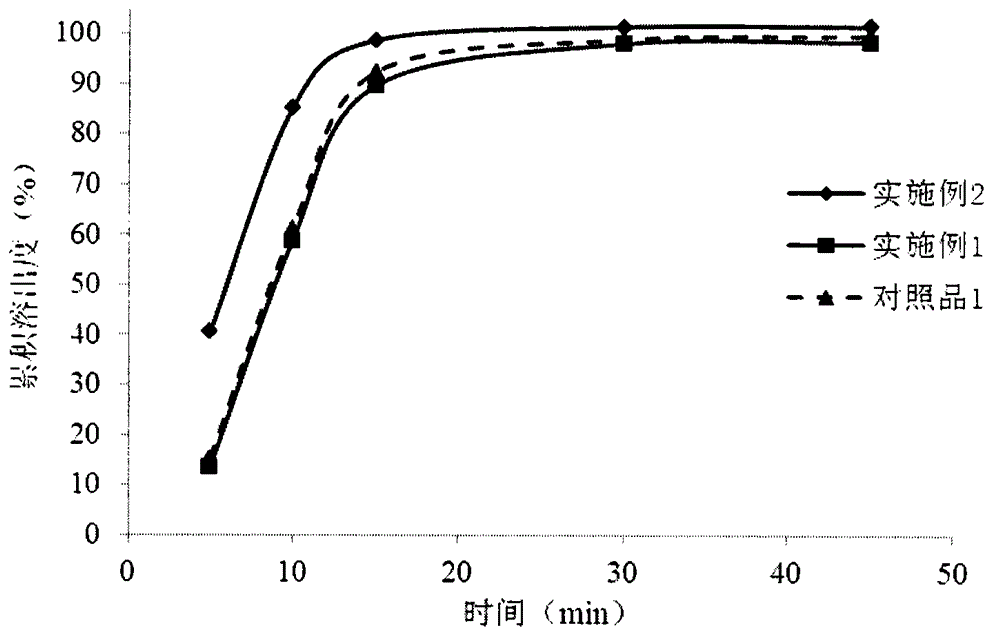
Image
Examples
Embodiment 1
[0023] active substance core
[0024] Example
active substance
50% ethanol
1
20
25
10
4
36
[0025] Isolation of Active Substance Cores
[0026] Example
Cores with active substances
80% ethanol
1
66
4
1
50
[0027] Preparation of organic acid pellet core
[0028] Example
organic acid
50% ethanol
1
20
25
10
4
36
[0029] Isolation of organic acid pellet cores
[0030] Example
Ball core containing organic acid
80% ethanol
1
66
4
1
50
[0031] Weigh 4g of polyvinylpyrrolidone and dissolve it in 36ml of 50% ethanol solution. 20g of dabigatran etexilate mesylate and 25g of microcrysta...
Embodiment 2
[0036] active substance core
[0037] Example
[0038] Isolation of Active Substance Cores
[0039] Example
[0040] Preparation of organic acid pellet core
[0041] Example
[0042] The preparation method is the same as in Example 1, except that the organic acid pellet core is not subjected to isolation coating. The active substance pellets and organic acid pellet cores are filled into the hard capsule shell in proportion, and a capsule machine with secondary filling function is selected for filling.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com