Double-effect optical Fenton denitrification method of manganese ferrite or carbon composite material of manganese ferrite
A technology of carbon composite materials and manganese ferrite, applied in the field of environmental science, can solve the problems of high cost, lower denitrification efficiency, and inability to make full use of solar visible light, and achieve the effect of low cost and improved photocatalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
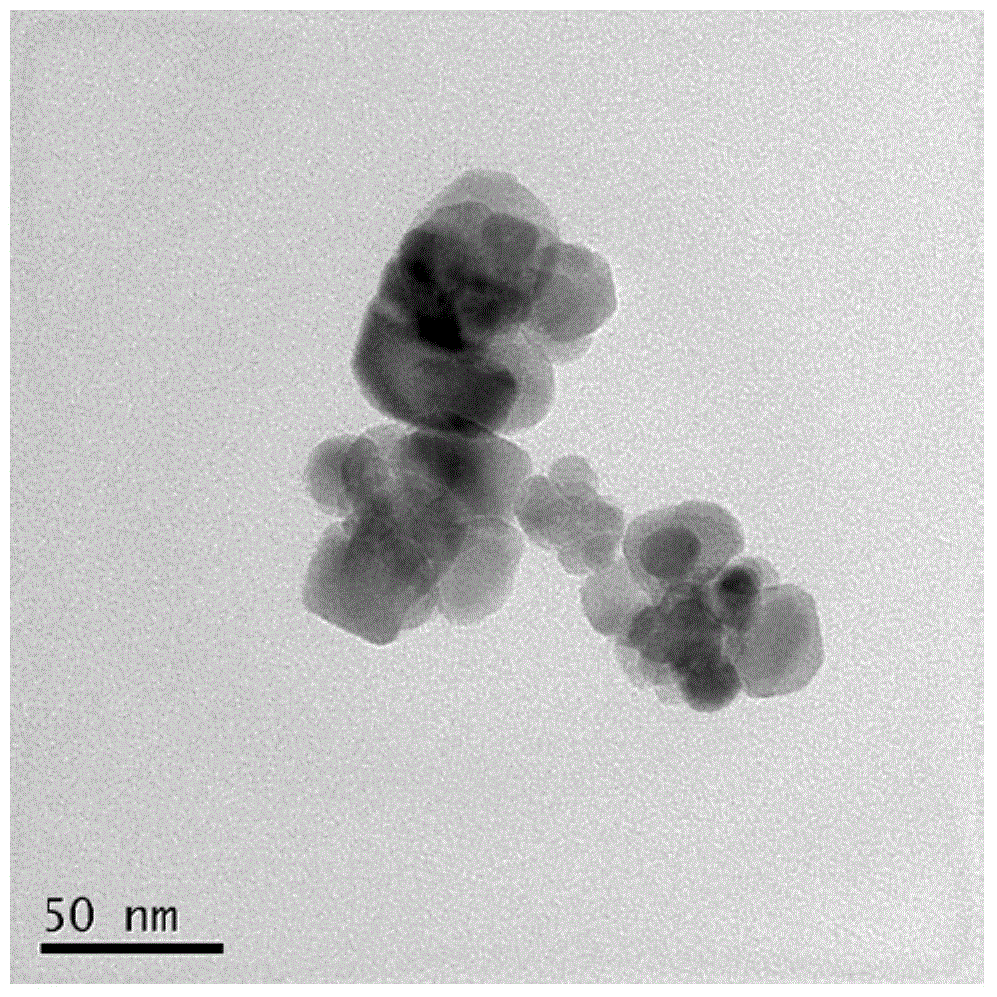
[0035] The synthesis of embodiment 1 manganese ferrite: weigh MnCl in molar ratio 1:2 2 .4H 2 O (0.01mol, 1.9790g), FeCl 3 .6H 2 O (0.02mol, 5.4058g) was dissolved in 15ml of ultrapure water respectively, and stirred for 10min under magnetic stirring to make it evenly mixed. Another accurately weighed NaOH (0.08mol, 3.2000g) was dissolved in 15ml of water. Under magnetic stirring, sodium hydroxide was added dropwise to the mixed solution of nickel sulfate hexahydrate and ferric chloride hexahydrate, and then the sodium hydroxide in the beaker was washed with ultrapure water and added to the mixed solution. Stir for 20 minutes to make it evenly mixed, and the total volume is about 60ml. Then add the mixed solution into two 50ml hydrothermal reaction kettles, place it at 180°C for 10 hours, after cooling, take it out and separate it in a magnetic field, wash it 3 times (washing with ultrapure water), and dry it at 200°C 4h, the sample MnFe is obtained 2 o 4 .
Embodiment 2
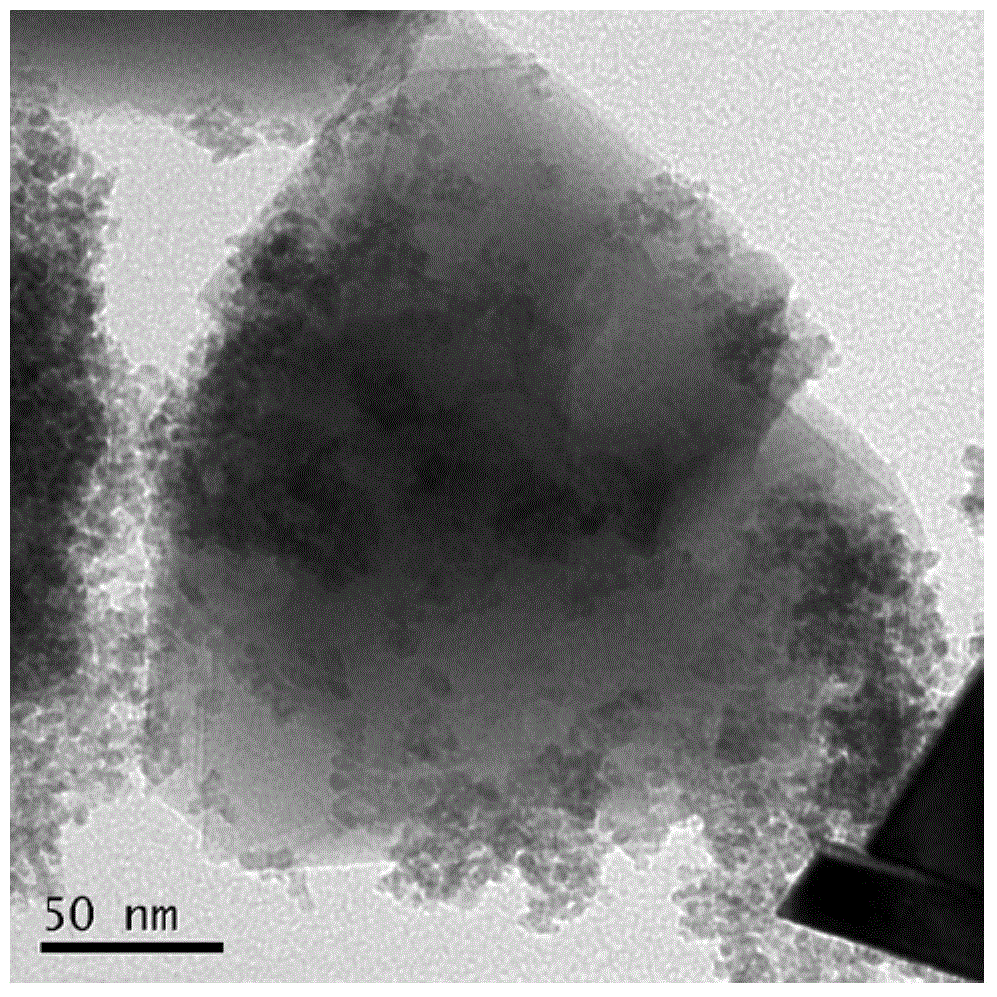
[0036] Example 2 Synthesis of activated carbon hybrid manganese ferrite catalyst: Weigh MnCl at a molar ratio of 1:2 2 .4H 2 O (0.01mol, 1.9790g), FeCl 3 .6H 2O (0.02mol, 5.4058g) was dissolved in 15ml of ultrapure water respectively, after mixing, 0.2344g of activated carbon was added, ultrasonicated for 30min, and then stirred by magnetic force for 10min to make it evenly mixed. Another accurately weighed (0.08mol, 3.20g) sodium hydroxide was dissolved in 15ml of water. Add sodium hydroxide dropwise to the mixed suspension under magnetic stirring, then wash the sodium hydroxide in the beaker with ultrapure water and add it to the mixed solution. Stir for 30 minutes to make it evenly mixed, and the total volume is about 60ml. Then add the mixed solution into a 100ml reaction kettle, place it at 180°C for 10 hours, cool it, take it out and separate it in a magnetic field, wash it 3 times (ultrapure water washing), and dry it at 200°C for 4 hours to get the activated carbon...
Embodiment 3
[0037] The synthesis of embodiment 3 graphene hybrid manganese ferrite catalysts:
[0038] The synthesis method of graphene oxide (GO): accurately weigh 2.0g graphite, 1.0g NaNO 3 In a 200ml beaker, add 50ml concentrated H 2 SO 4 Then add 6.0g KMnO 4 React for 2h under the condition that the temperature is lower than 20°C. Then warm up to 35 o C, add a certain amount of deionized water to the reaction system after 35 minutes of reaction, keep stirring for 20 minutes, and then use 5% H 2 o 2 Reduce the remaining oxidant until the solution turns bright yellow, filter while hot, and wash with 5% HCl and deionized water until there is no SO in the solution 4 2- , fully dried in a vacuum oven at 60°C to prepare a graphite oxide sample for future use.
[0039] Preparation of graphene hybrid manganese ferrite: Accurately weigh graphite oxide with 4% of the theoretical mass of manganese ferrite and ultrasonically disperse it in 10ml of water for later use. Weigh MnCl at a mo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com