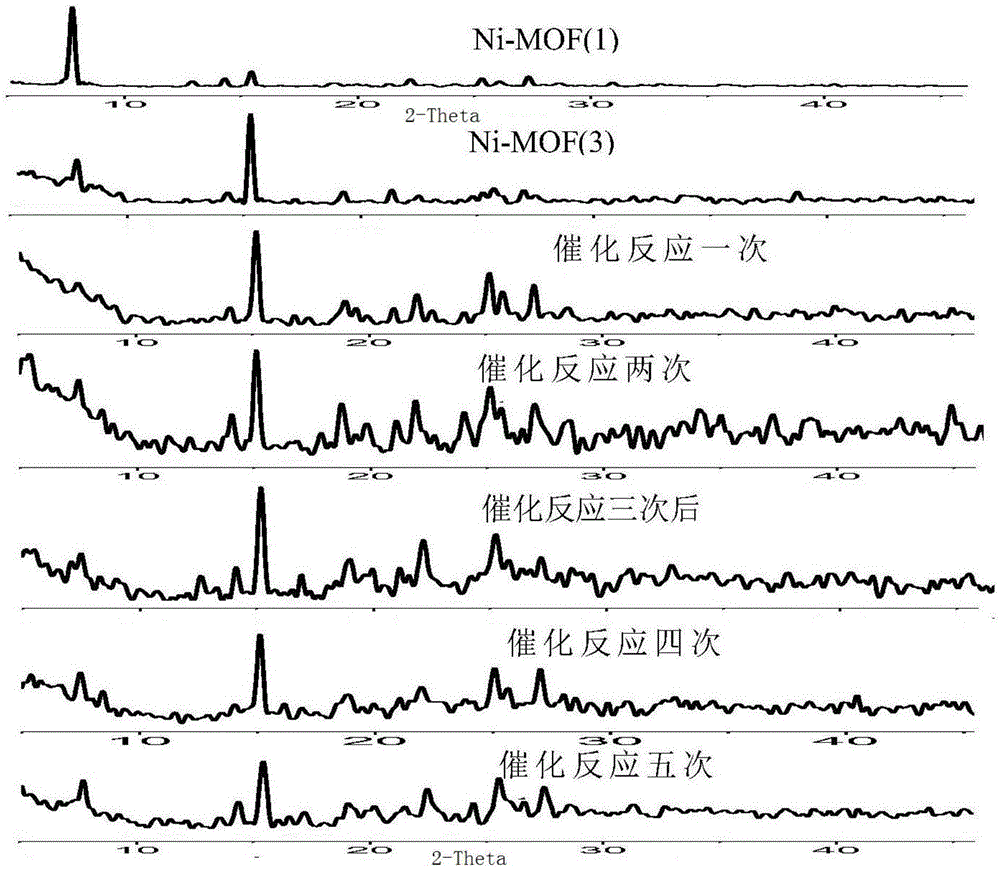
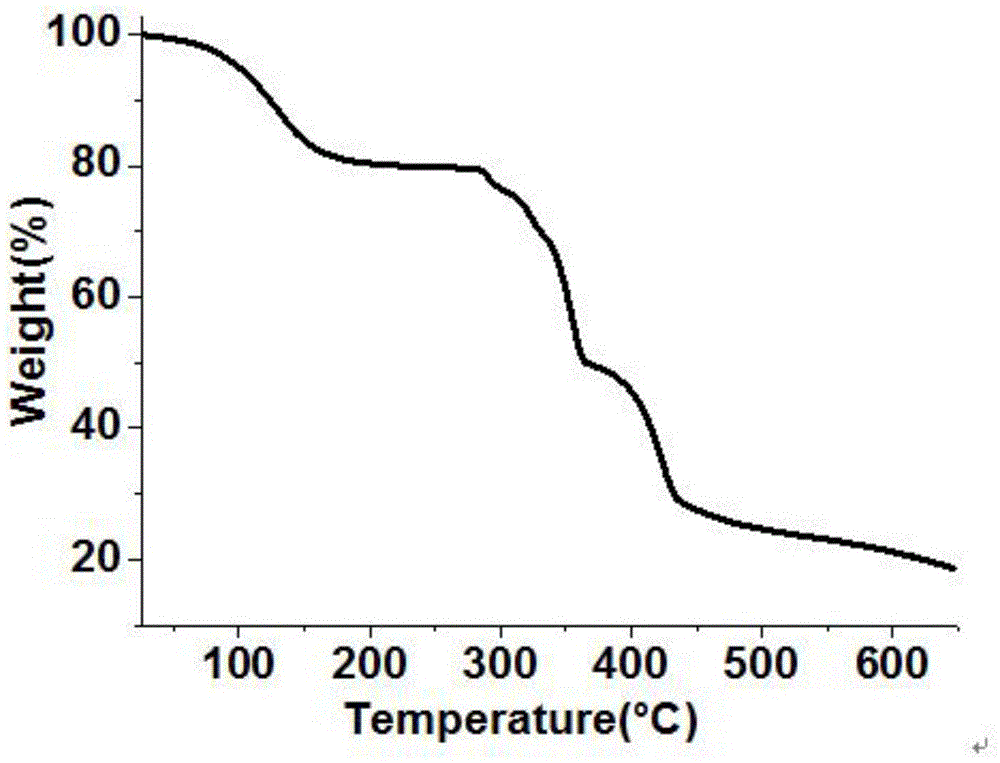
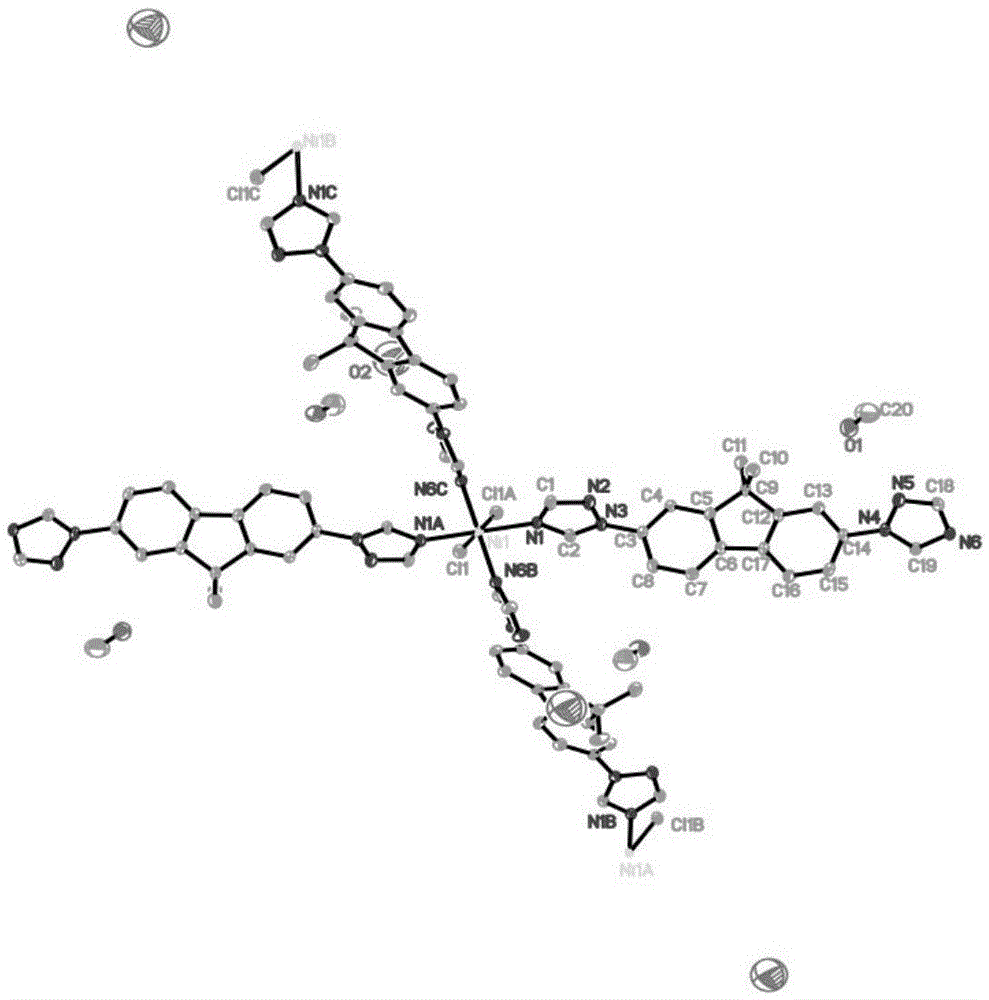
Ni(II) metal-organic frameworks and their synthesis methods and applications
A metal-organic framework, ni-mof-3 technology, used in nickel-organic compounds, organic chemistry, organic compounds/hydrides/coordination complex catalysts, etc., which can solve the problems of long running time, strong toxicity, and low catalytic efficiency question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] The synthesis route of the catalyst synthesized by the present invention is as follows:
[0065] 1. Preparation of Ligand L:
[0066]
[0067] At room temperature, 2,7-dibromofluorene (10.40g, 32mmol), tetrabutylammonium bromide (0.10g, 0.3mmol), methyl iodide (9.93g, 90mmol), and 10mL of 50% NaOH solution were added to 140mL of In DMSO, ultrasonic reaction (100W) was performed for 5 hours. After the reaction was completed, it was poured into 500mL water, stirred for 20min and left to stand for 1 hour, filtered with suction, and the filter cake was washed with 1% NaCl aqueous solution and dried to obtain 10.97g of a yellow solid. The yield was 97.40%.
[0068] N 2 Under protection, intermediate A (3.52g, 10mmol) 1,2,4-triazole (1,93g, 28mmol), cesium carbonate (13.03g, 40mmmol, cuprous iodide (0.762g, 4mmol) were placed in 100ml In the there-necked flask, 20mlDMF was used as solvent, heated to 120°C, tracked by TLC, poured into 300ml of water after the reaction ...
experiment example 1
[0076] Experimental Example 1 Benzaldehyde reacts with trimethylsilyl cyanide to generate 2-phenyl-2-(trimethylsilyloxy) acetonitrile
[0077] At room temperature, benzaldehyde (106mg, 1mmol) and trimethylsilyl cyanide (119mg, 1.2mmol) were added to a 25ml round bottom flask, and then 32.5mg (0.033mmol) of the catalyst Ni-MOF-3 was added, and the reaction was stirred. 1 After the HNMR tracking reaction, 1.5 ml of dichloromethane was added, and the catalyst was recovered by quick centrifugation, and the liquid was used to calculate the yield by GC-MS.
[0078] Purification of the product (2-phenyl-2-(trimethylsilyloxy)acetonitrile): evaporate the centrifuged liquid to dryness under reduced pressure, dissolve it in n-hexane, perform silica gel column chromatography (n-hexane:ethyl acetate=9:1 ) to obtain a colorless oily product. That 1 HNMR such as Figure 14 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com