A kind of synthetic method of stable isotope labeling beta receptor agonist compound
A stable isotope and agonist technology, applied in the field of preparation of isotope-labeled compounds, can solve the problems of complicated operation, high price, difficult control of isotope abundance, etc., and achieve the effect of easy purification, few by-products, and good promotion prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
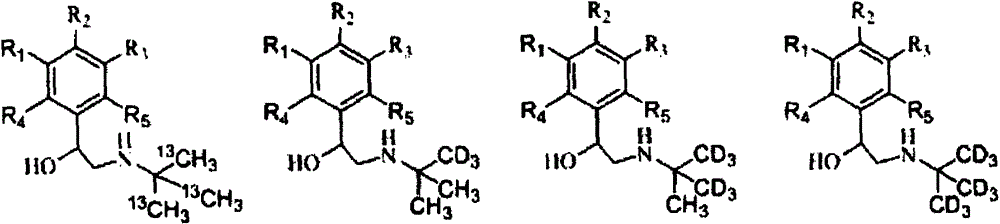
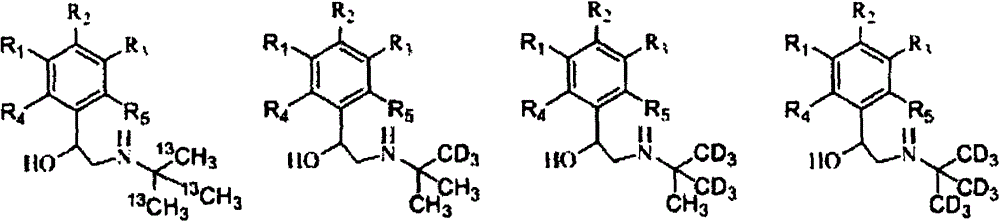
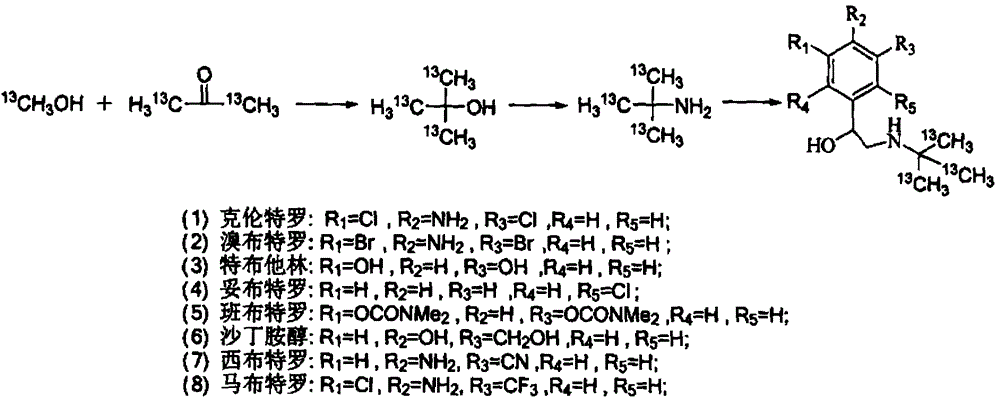
[0040] A method for synthesizing stable isotope-labeled β-receptor agonist compounds, characterized in that the method adopts the following steps:
[0041] (1) Add acetone or stable isotope-labeled acetone dropwise to stable isotope-labeled methanol at low temperature, and control the molar ratio of stable isotope-labeled methanol to acetone or stable isotope-labeled acetone to be 1:0.5~1:3. React at room temperature for 0.5-24 hours, quench the reaction with dilute hydrochloric acid to obtain stable isotope-labeled tert-butanol, and then ammoniate with an ammoniating reagent to obtain stable isotope-labeled tert-butylamine;
[0042] (2) Bromoketones are used as the precursors of β-receptor agonist compounds, which undergo condensation reaction with stable isotope-labeled tert-butylamine in a liquid-phase environment, and are then reduced by a reducing agent to obtain stable isotope-labeled β-receptors Agonist compounds.
[0043] Step (1) When adding acetone or stable isotope...
Embodiment 1
[0065] A. Stable isotope labeled tert-butylamine-D 9 Synthesis
[0066] In a 100mL flask with a thermometer and a condenser tube, add 3.607g (0.1mol) of methanol-D 4 , lower the temperature to 0°C, and slowly add 38.071g (0.15mol) of iodine and an appropriate amount of red phosphorus in batches under stirring conditions. After the reaction is completed, distill the obtained iodomethane-D 3 , and then utilize the methyl iodide-D prepared above 3 Prepare Grignard reagent, slowly add 6.412g (0.1mol) acetone-D dropwise at -5°C 6 , react at room temperature for 0.5h, quench the reaction with dilute hydrochloric acid, and obtain tert-butanol-D 9 . After the obtained tert-butanol was purified by rectification, the reaction temperature was controlled at -10°C, and 7.059g (0.15mol) of methoxyamine was added dropwise to tert-butanol-D 9 Neutralize the catalyst, continue to react for 2 hours after the dropwise addition, and the reaction solution is rectified to obtain tert-butylamin...
Embodiment 2
[0070] A. Stable isotope labeled tert-butylamine- 13 C 3 Synthesis
[0071] In a 100mL flask with a thermometer and a condenser tube, add 3.305g (0.1mol) of methanol- 13 C, lower the temperature to 0°C, and slowly add 38.071g (0.15mol) of iodine and an appropriate amount of red phosphorus in batches under stirring conditions. After the reaction is completed, distill the obtained methyl iodide- 13 C, and then utilize the methyl iodide prepared above- 13 C was prepared as Grignard reagent, and slowly added dropwise 6.081g (0.1mol) of acetone at 5°C- 13 C 2, react at room temperature for 3h, quench the reaction with dilute hydrochloric acid, and obtain tert-butanol- 13 C 3 . After the obtained tert-butanol was purified by rectification, the reaction temperature was controlled at -10°C, and 7.722g (0.15mol) of imine chloride was added dropwise to tert-butanol- 13 C 3 Neutralize the catalyst, continue to react for 2 hours after the dropwise addition, and the reaction solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com