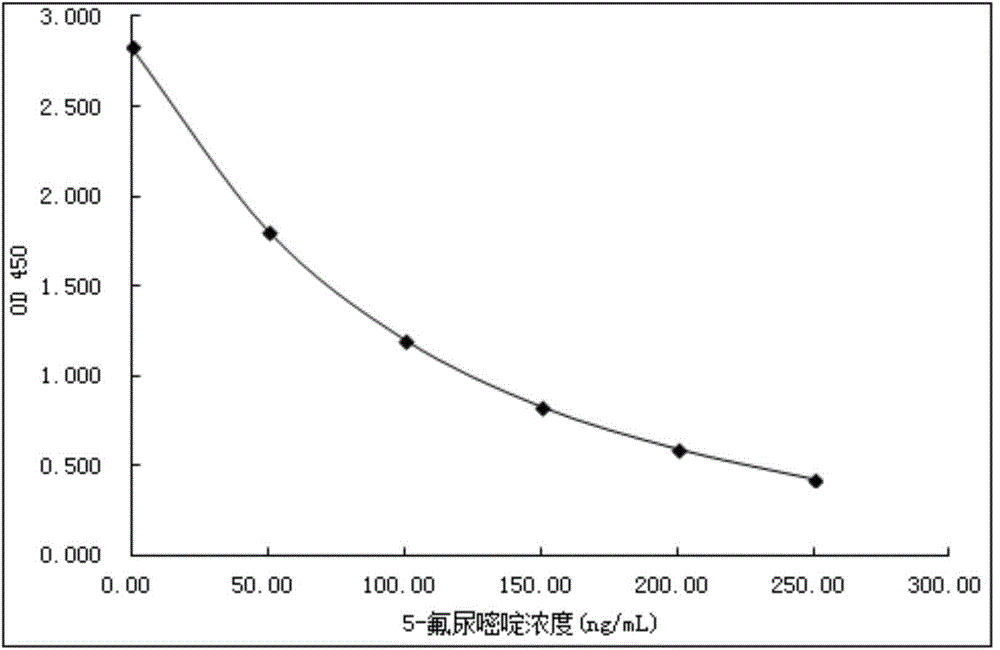
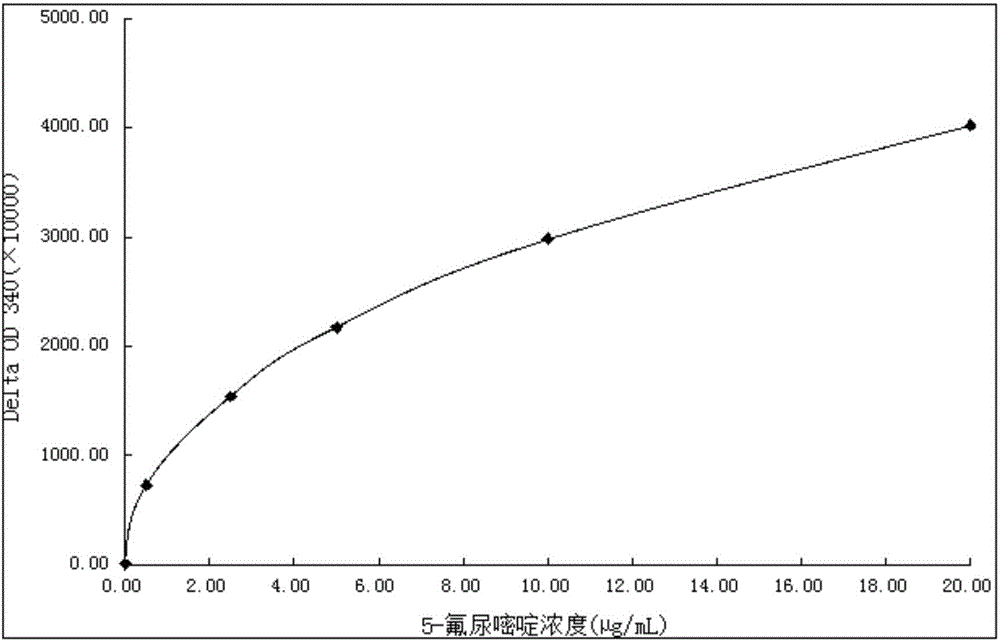
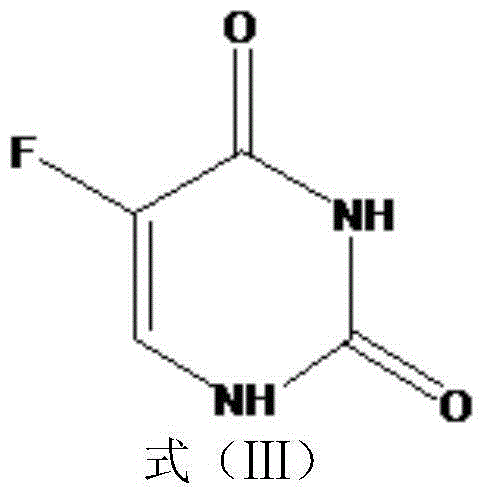
5-fluorouracil derivatives, 5-fluorouracil immunogens, antibodies for immunogens and 5-fluorouracil detection kit
A technology of fluorouracil and derivatives, applied in immunoglobulins, animal/human peptides, animal/human proteins, etc., can solve the problems of not meeting the needs of clinical testing and not being suitable for clinical applications.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Synthesis and structure confirmation of 5-fluorouracil derivatives
[0040] The chemical structure of the 5-fluorouracil derivative used in the following examples is shown in formula (IV):
[0041]
[0042] The synthetic route of 5-fluorouracil derivative shown in this formula (IV) is as follows:
[0043]
[0044] The synthetic steps of the 5-fluorouracil derivative shown in formula (IV) are as follows:
[0045] 1) Weigh 5g (38.5mmol) of compound 15-fluorouracil, 6.95g (50.3mmol) of 2-bromoacetic acid and 4.48g (80.0mmol) of KOH and dissolve them in 50mL of water, then stir the solution at 60°C overnight; The reactant was adjusted to pH=5 with HCl, and then filtered; the filtered solid was washed with water and then vacuum-dried to obtain 2 g of 5-fluorouracil derivative as a white solid, with a yield of 27.6%.
[0046] 2) Structural identification of the purified product obtained above:
[0047] a. Using Varian III plus 300MHz to scan the NMR spectr...
Embodiment 2
[0050] Example 2: Synthesis of BSA-5-fluorouracil derivative immunogen
[0051] BSA-5-fluorouracil immunogen is composed of bovine serum albumin (Bovine Serum Albumin, BSA) and 5-fluorouracil derivative-(CH 2 ) n-COO-group connected, in this embodiment, take n=1 as an example to describe the synthesis method of the immunogen in detail, the specific steps are as follows:
[0052] Bovine serum albumin (200mg) was dissolved in 50ml 0.2M, in the phosphate buffer of pH 8.5;
[0053] Add the following chemicals into a small beaker and stir to dissolve: 200 mg of synthetic 5-fluorouracil derivative, 3.5 ml of dimethylformamide (dimethylformamide, DMF), 3.5 ml of ethanol, 7.0 ml of 10 mM potassium phosphate buffer at pH 5.0, 220mg 1-ethyl-3-(-3-dimethylaminopropyl) carbodiimide, 50mg N-hydroxysulfosuccinimide (N-hydroxysulfosuccinimide, Sulfo-NHS), these chemicals at room temperature Stir and dissolve for 30 minutes;
[0054] The dissolved solution was added dropwise to the BSA sol...
Embodiment 3
[0055] Example 3: Synthesis of KLH-5-fluorouracil derivative immunogen
[0056] KLH-5-fluorouracil immunogen is composed of hemocyanin (KLH) and 5-fluorouracil derivative-(CH 2 )n-COO-group connected, in this embodiment, take n=2 as an example to describe the synthesis method of the immunogen in detail, the specific steps are as follows:
[0057] Hemocyanin (180mg) was dissolved in 60ml 0.15M, in the phosphate buffer of pH 8.7;
[0058] Add the following chemicals into a small beaker and stir to dissolve: 200 mg of synthetic 5-fluorouracil derivatives, 3.5 ml of dimethylformamide (dimethylformamide, DMF), 2.8 ml of ethanol, 6.3 ml of 12 mM potassium phosphate buffer at pH 4.8, 160mg 1-ethyl-3-(-3-dimethylaminopropyl) carbodiimide, 40mg N-hydroxysulfosuccinimide (N-hydroxysulfosuccinimide, Sulfo-NHS), put these chemicals at room temperature Stir and dissolve for 25 minutes;
[0059] The dissolved solution was added dropwise to the KLH solution and stirred overnight at 2°C to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com