Phosphomannose isomerase gene with plant origin and application of phosphomannose isomerase gene
A mannose phosphate, plant-derived technology, applied in the isolation, cloning and application of mannose phosphate isomerase gene ChlaPMI, can solve safety concerns, the safety of PMI has not attracted enough attention, and the adverse effects of transforming the recipient genome And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
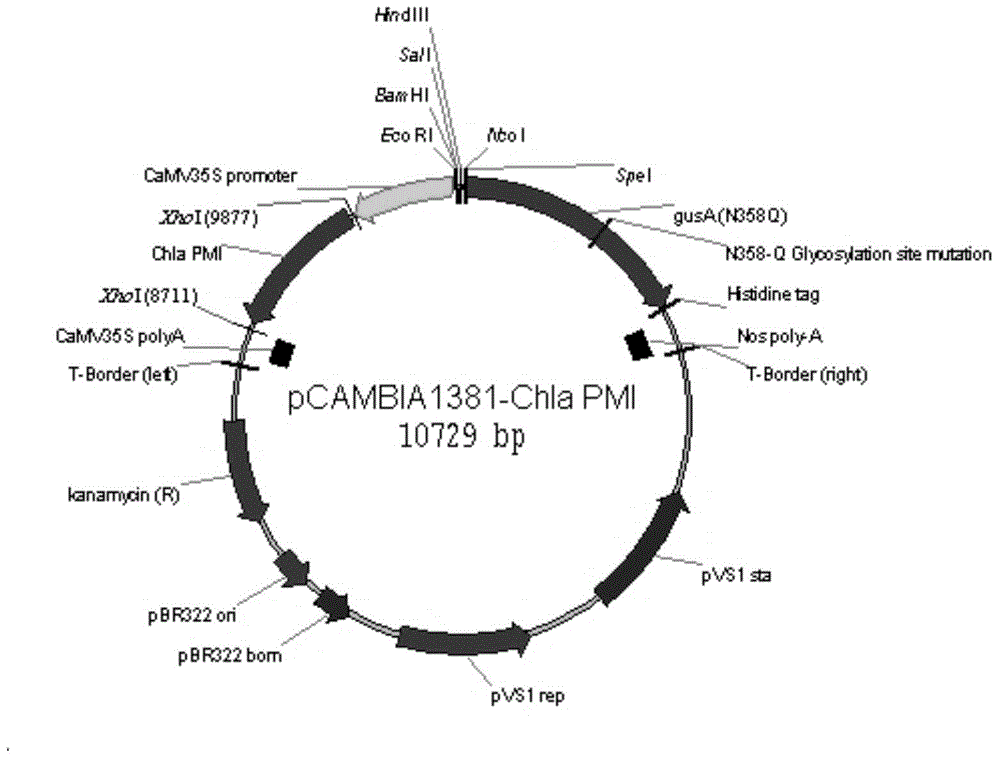
Image
Examples
Embodiment 1
[0037] Embodiment 1 - the acquisition and cloning of ChlaPMI gene
[0038]The sequence is based on the bacterial PMI protein sequence, compared with the homologous sequence in the genome sequence of Chlamydomonas that can utilize mannose (www.ncbi.nlm.nih.gov), and the one with the highest homology is ChlaPMI protein sequence. The RNA of Chlamydomonas reinhardtii was extracted and reverse-transcribed into cDNA; according to the CDS (coding sequence) sequence of ChlaPMI, gene-specific cloning primers were designed, forward primer 5'-ATGCTGCAGCTTCGCTGTGCTG-3', reverse primer 5'-CACCTCCGCCTCTCCAGCCGTC-3'; PCR amplification was performed using cDNA as a template.
[0039] Recover the target fragment amplified by PCR, the target fragment length is 1095bp, connect it to the PGEM-T-Easy carrier (purchased from Promega Company, operate according to the instruction manual of the carrier), and transform Escherichia coli XL-Blue competent cells according to the heat shock method; Then,...
Embodiment 2
[0041] Embodiment 2——The construction of the prokaryotic expression vector of ChlaPMI gene
[0042] By designing ChlaPMI prokaryotic expression primers, the forward primer 5'- GGATCC ATGCTGCAGCTTCGCTGTGCTG-3' (the underline is the BamHI restriction site), reverse primer 5'- CTCGAG CTCCGCCTCTCCAGCCGTC-3' (the underline is the XhoI restriction site), using the PGEM-T-ChlaPMI recombinant plasmid as a template, carry out PCR amplification, recover the target fragment amplified by PCR and digest pGEX-6P-1 with BamHI and XhoI for expression The vector (purchased from GE) was ligated to obtain the prokaryotic expression vector pGEX-ChlaPMI fused with the GST (glutathione-S-transferase) fragment, and transformed into Escherichia coli expression strain BL21. At the same time, the pGEX-6P-1 empty vector and the pGEX-PMI containing the Escherichia coli PMI expression vector were also transformed into the Escherichia coli expression strain BL21.
Embodiment 3
[0043] Embodiment 3——ChlaPMI activity analysis
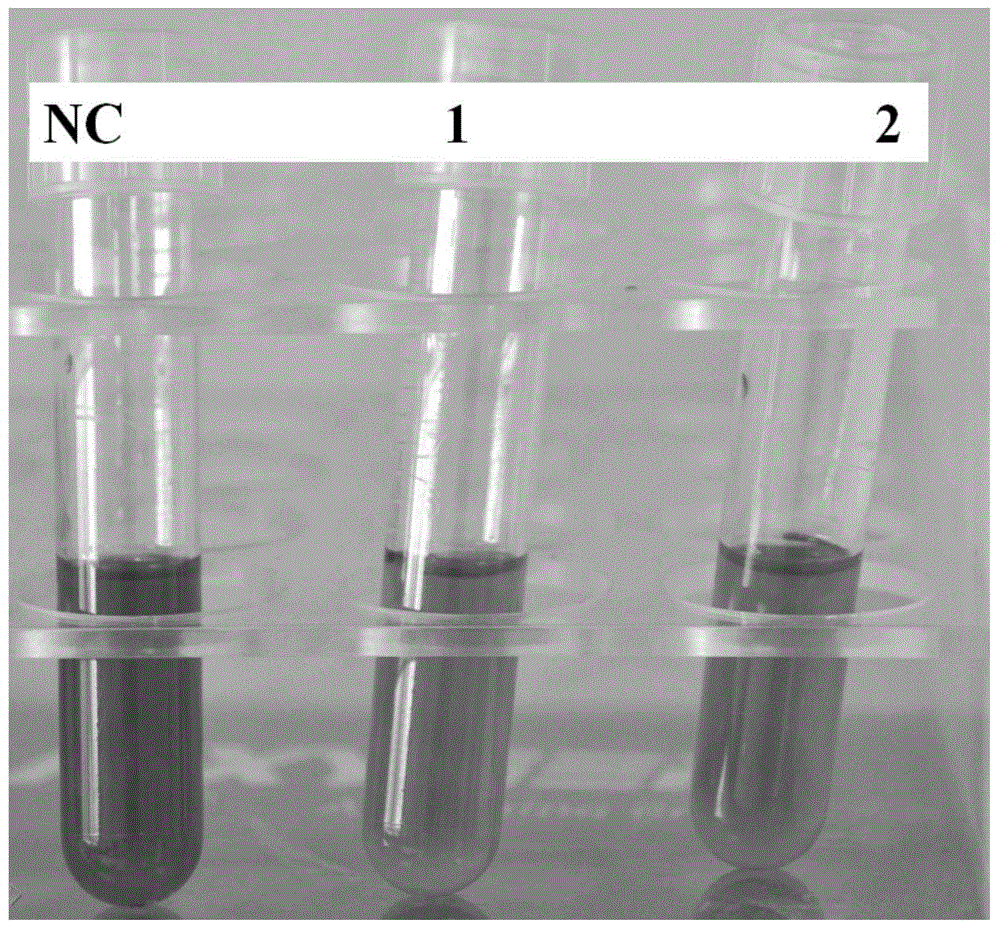
[0044] The BL21 bacterial strain containing the prokaryotic expression vectors pGEX-ChlaPMI, pGEX-PMI and pGEX-6P-1 empty vector was drawn, and a single clone was picked to inoculate the LB liquid medium (see Table 1 for the Agrobacterium culture medium without adding agar), Shake culture at 37°C overnight (200r / min). The next day, centrifuge at 6000r / min for 1min at room temperature, discard the supernatant, and resuspend the pellet with a small amount of sterile water. Take the resuspension and inoculate it into sterile phenol red chromogenic medium (1% peptone, 0.5% sodium chloride, 50mg / L phenol red, 30% mannose, pH 7.4) at a ratio of 1:50, and culture with shaking at 37°C ( 200r / min), observe the color change of the medium after 48h. If the strain has the ability to metabolize mannose, the medium will be acidified, the pH value will drop, and the color of the medium will gradually change from red at pH 7.4 to yellow. For...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com