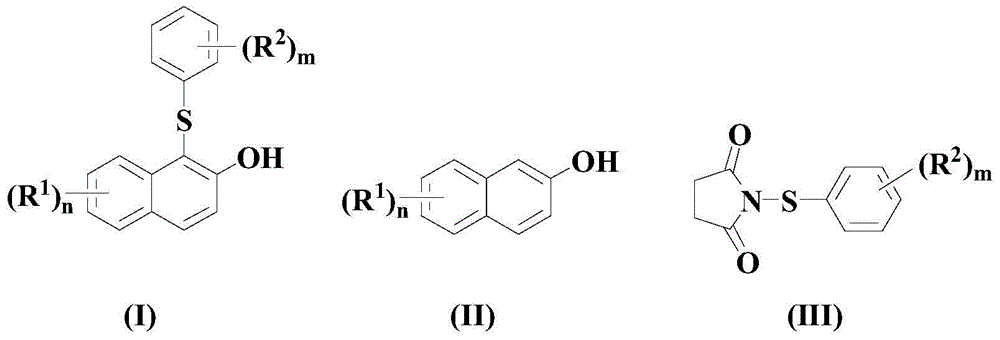
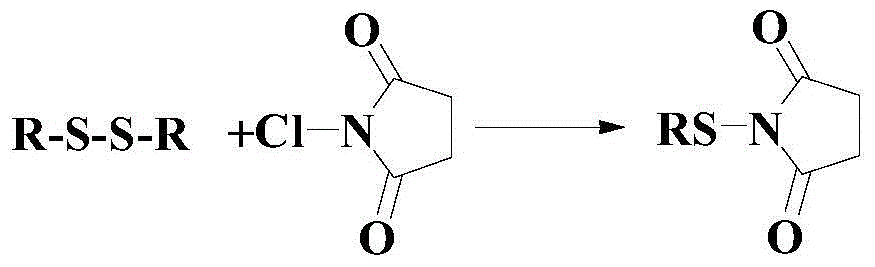
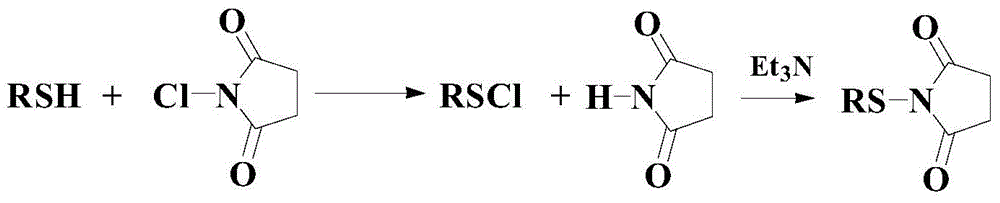A kind of synthetic method of intermediate thiosuccinimide compound in pharmaceutical field
A technology of thiosuccinimide and synthesis method, applied in directions such as organic chemistry, can solve the problems of low product yield, increased reaction flow and the like, and achieve the effects of low price and good industrial production potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048]
[0049] At room temperature, in an appropriate amount of organic solvent dimethylsulfoxide (DMSO), add 100mmol formula (II) compound bromobenzene, 100mmol formula (III) compound N-bromosuccinimide, 150mmol formula (IV) compound vulcanization Ammonium, 200mmol base 2-methylpiperidine, 4mmol catalyst (as 3mmol copper trifluoromethanesulfonate (Cu(OTf) 2 ) and 1 mmol of tetrakis(triphenylphosphine) palladium), the temperature was raised to 70° C., and the reaction was stirred at this temperature for 15 hours, and the whole reaction process was carried out under nitrogen atmosphere.
[0050] After the reaction is finished, place it, let it cool naturally to room temperature, fully wash with deionized water, separate layers, get the upper layer with a camera, remove the solvent under reduced pressure, and the residue is subjected to flash column chromatography (the eluent used in flash column chromatography is dichloro The mixture of methane and petroleum ether, wherein ...
Embodiment 2
[0054]
[0055] At room temperature, in an appropriate amount of organic solvent dimethylsulfoxide (DMSO), add 100mmol formula (II) compound p-bromotoluene, 150mmol formula (III) compound N-bromosuccinimide, 200mmol formula (IV) compound Ammonium sulfide, 300mmol base 2-methylpiperidine, 7mmol catalyst (as 5mmol copper trifluoromethanesulfonate (Cu(OTf) 2 ) and 2 mmol of tetrakis(triphenylphosphine) palladium), the temperature was raised to 90° C., and the reaction was stirred at this temperature for 12 hours, and the whole reaction process was carried out under nitrogen atmosphere.
[0056] After the reaction is finished, place it, let it cool naturally to room temperature, fully wash with deionized water, separate layers, get the upper layer with a camera, remove the solvent under reduced pressure, and the residue is subjected to flash column chromatography (the eluent used in flash column chromatography is dichloro The mixture of methane and petroleum ether, wherein the ...
Embodiment 3
[0060]
[0061] At room temperature, in an appropriate amount of organic solvent dimethylsulfoxide (DMSO), add 100mmol formula (II) compound o-bromonitrobenzene, 200mmol formula (III) compound N-bromosuccinimide, 250mmol formula (IV ) compound ammonium sulfide, 400mmol base 2-methylpiperidine, 10mmol catalyst (7.5mmol copper trifluoromethanesulfonate (Cu(OTf) 2 ) and 2.5 mmol of tetrakis(triphenylphosphine) palladium), the temperature was raised to 110° C., and the reaction was stirred at this temperature for 8 hours, and the whole reaction process was carried out under nitrogen atmosphere.
[0062] After the reaction is finished, place it, let it cool naturally to room temperature, fully wash with deionized water, separate layers, get the upper layer with a camera, remove the solvent under reduced pressure, and the residue is subjected to flash column chromatography (the eluent used in flash column chromatography is dichloro The mixture of methane and petroleum ether, wher...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com