Acidic xylanase mutant and application thereof
A technology of xylanase mutation and xylanase, which is applied in the field of acid xylanase mutants and can solve the problem of less acid xylanase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The amplification of embodiment 1 xylanase gene
[0025] According to the gene sequence in the public gene database, the codon of the synthetic gene was optimized and the acid xylanase gene XynH2 was artificially synthesized. Its nucleotide sequence is SEQ ID NO: 2, and its encoded amino acid sequence is SEQ ID NO: 1 .
[0026] The acid xylanase gene XynH2 fragment was cloned by PCR reaction, and the primers and reaction conditions were as follows:
[0027] Primer 1 (F): GCGCGAATTCTTTCCTTCTGAGTTGGCTCAA
[0028] Primer 2 (R): TAAAGCGGCCGCTTAAGAGACGGTAATAGTAGA
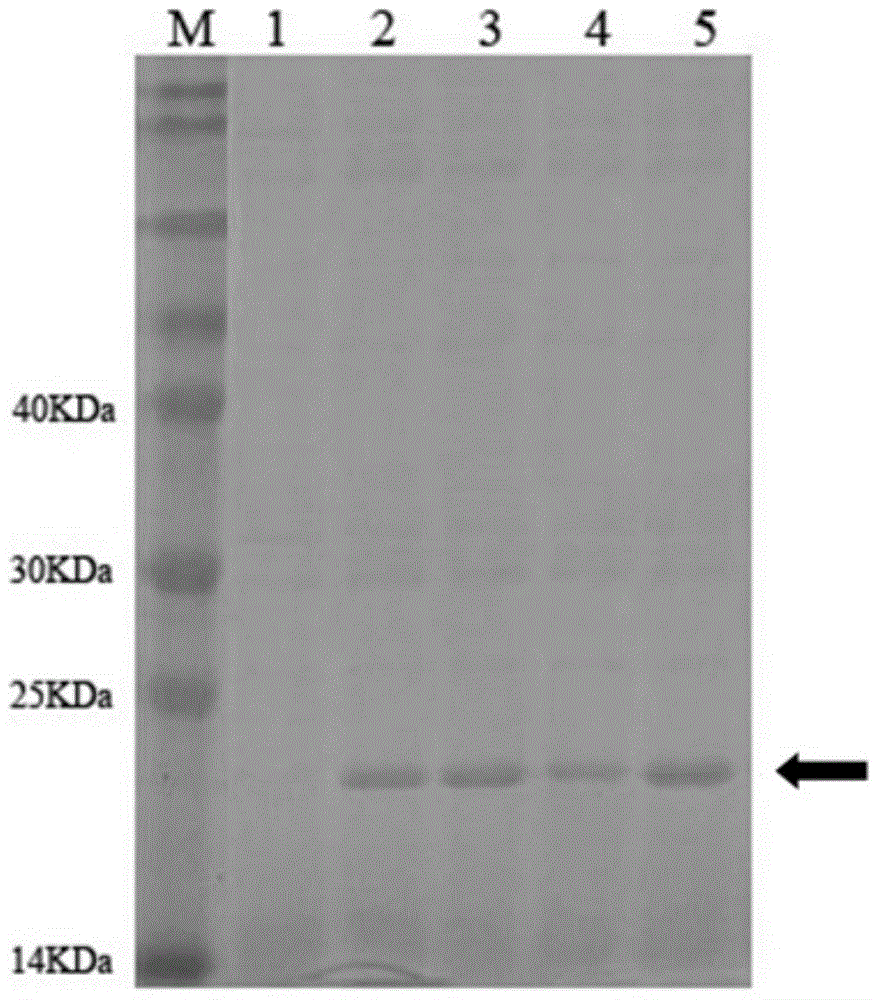
[0029] The reaction conditions were: denaturation at 94°C for 30s, renaturation at 56°C for 30s, extension at 72°C for 45s, and after 30 cycles, incubation at 72°C for 10 minutes. The results of agarose electrophoresis showed that the XynH2 gene was a 621bp fragment.
Embodiment 2
[0030] Construction and screening of embodiment 2 xylanase mutants
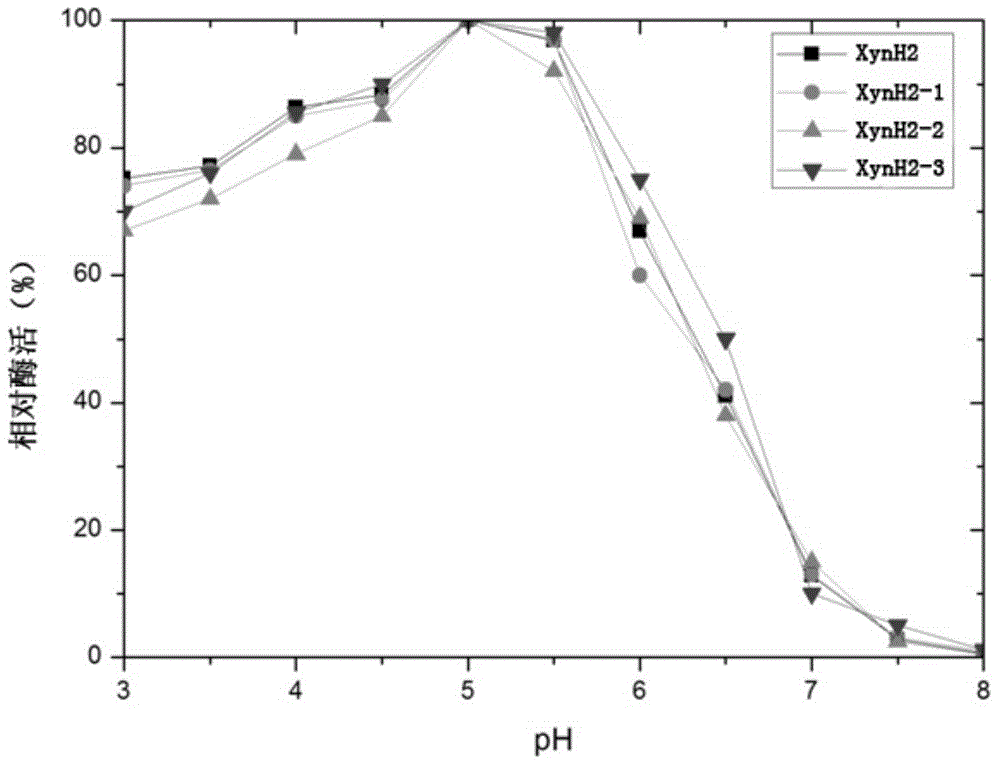
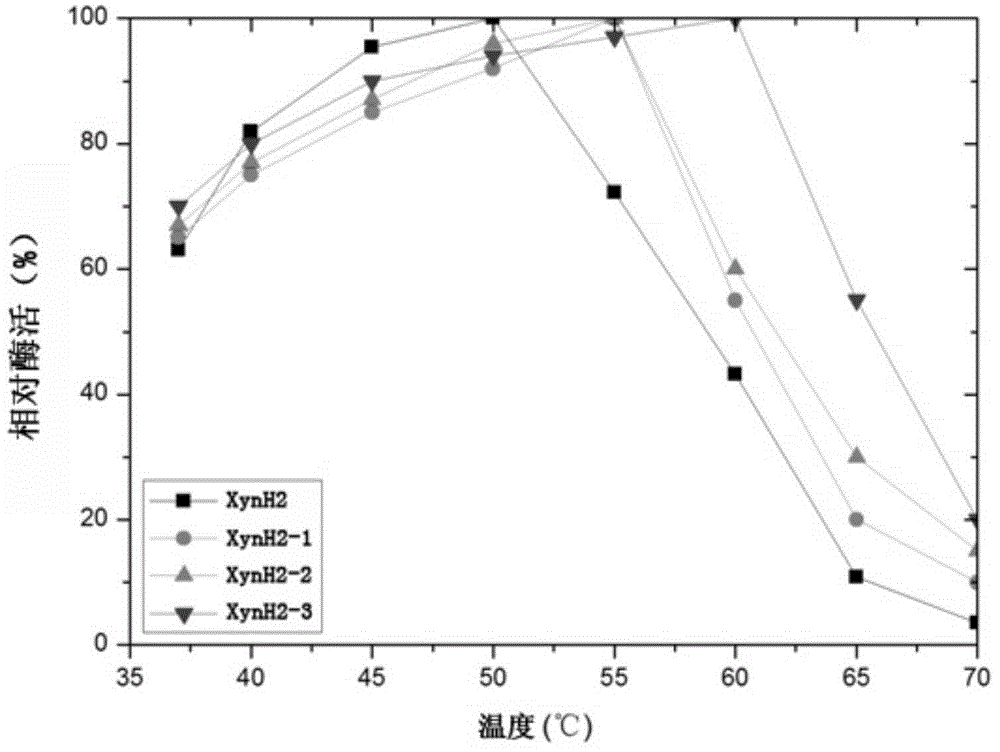
[0031] In order to improve the heat resistance of the above-mentioned xylanase XynH2, the applicant screened a large number of mutations of the enzyme by directed evolution technology.
[0032] Using the XynH2 gene as a template, use primers 1 and 2 to perform PCR amplification with the GeneMorph II Random Mutation PCR Kit (Stratagene), recover the PCR product from the gel, perform enzyme digestion with EcoRI and NotI, and then ligate it to the pET21a vector after the same enzyme digestion , transformed into Escherichia coli BL21(DE3), spread on LB+Amp plates, and culture them upside down at 37°C. After the transformants appeared, pick them one by one with a toothpick to a 96-well plate, and add 150ul of 0.1mM IPTG to each well. LB+Amp medium, cultivated at 37°C and 220rpm for about 6 hours, centrifuged to discard the supernatant, resuspended the bacteria with pH 5.5 buffer, and repeatedly freeze-thawed to br...
Embodiment 3
[0039] The construction of embodiment 3 pichia pastoris engineering strain
[0040]The wild-type xylanase gene XynH2 and the xylanase mutant gene XynH2-1, XynH2-2, and XynH2-3 fragments cloned above are connected to the expression vector pPIC9K through the EcoR I and Not I sites respectively, The recombinant expression vectors pPIC9K-XynH2, pPIC9K-XynH2-1, pPIC9K-XynH2-2, pPIC9K-XynH2-3 were constructed.
[0041] The expression vector was linearized with Sal I, and the linearized fragment of the expression plasmid was transformed into Pichia pastoris GS115 by electroporation, and the recombinant strains of Pichia pastoris GS115 / pPIC9K-XynH2, GS115 / pPIC9K-XynH2-1, GS115 / pPIC9K-XynH2-1, GS115 / pPIC9K-XynH2-2, GS115 / pPIC9K-XynH2-3, and then screen multiple copies of positive transformants on YPD plates containing different concentrations of geneticin.
[0042] The positive transformants screened were named as Pichia pastoris XynH2 (Pichia pastorisXynH2), Pichia pastoris XynH2-1 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com