A method for screening antioxidant active ingredients
A technology of anti-oxidation activity and thin-layer plate, which is applied in the direction of analyzing materials through chemical reactions, material analysis through observing the influence of chemical indicators, and preparation of test samples, etc., to achieve easy identification, memory, and operation Simple, low-cost experimental results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
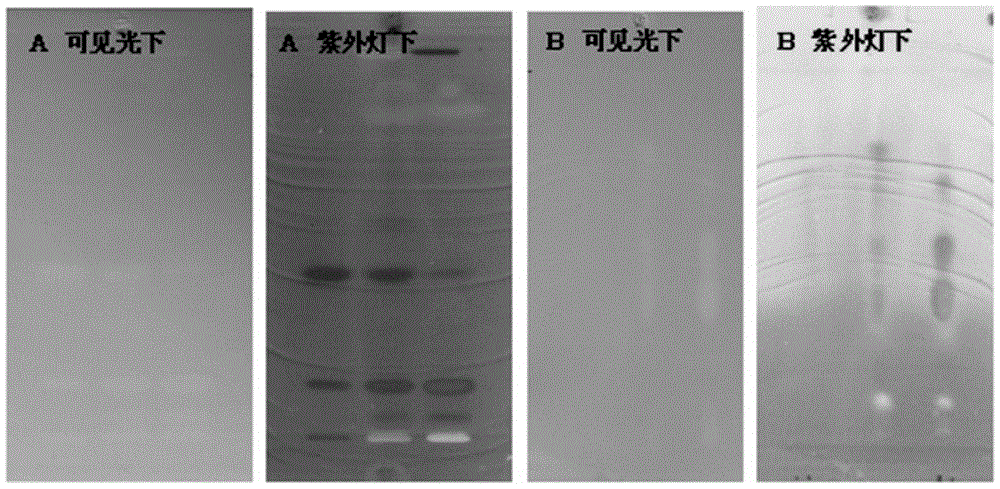
[0036] TLC plate A: Take 1g of perilla seed powder, weigh it accurately, add 5mL of methanol, and after ultrasonication for 25 minutes, take the supernatant and filter, and the filtrate is used as the test solution; take rosmarinic acid and luteolin reference substances separately , add methanol to make a solution containing 1mg per 1mL, as the reference solution; draw 1 μL of the reference solution and 10 μL of the test solution respectively, and spot the samples on the same silica gel GF254 thin-layer plate. After the methanol is evaporated, use n-hexane- Dichloromethane-ethyl acetate-formic acid (volume ratio: 2:5:2.5:0.5) is used as the developer for development; take it out and dry it for later use.
[0037] TLC plate B: Take 0.5g of Cistanche deserticola powder, weigh it accurately, add 10mL of methanol, take the supernatant after ultrasonication for 25 minutes, filter, and use the filtrate as the test solution; take another ergosteroside reference substance, add methanol...
Embodiment 2
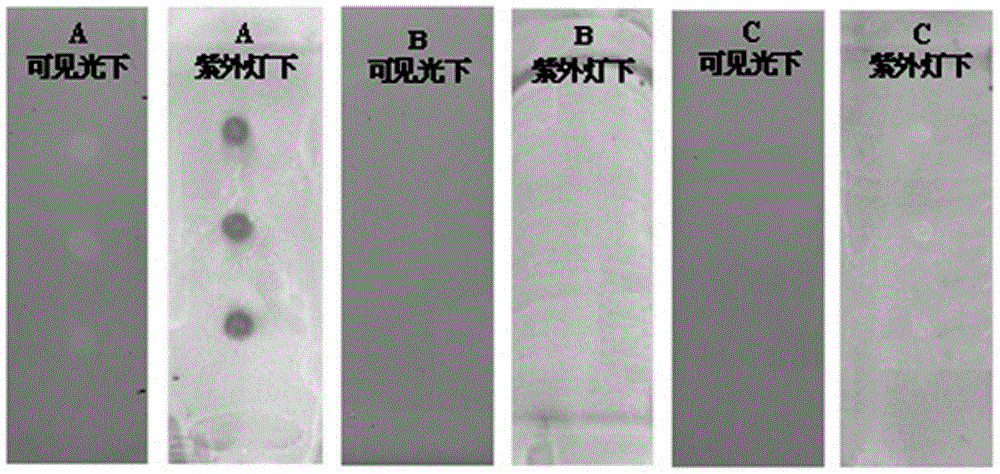
[0043] TLC plate A: Accurately weigh 1 mg of water-soluble vitamin E (Trolox), dissolve it in 1 mL of absolute ethanol, and prepare a solution with a concentration of 1 mg / mL; draw 2 μL of the solution and spot on silica gel GF 254 On the TLC plate A, shake off the ethanol and set aside.
[0044]TLC plate B: Accurately weigh 1 mg β-sitosterol, dissolve it in 1 mL of absolute ethanol, and prepare a solution with a concentration of 1 mg / mL; absorb 2 μL of the solution and apply it on silica gel GF254 thin-layer plate B, shake off the ethanol for later use .
[0045] Thin-layer plate C: Accurately weigh 1mg of nuciferine, dissolve it in 1mL of absolute ethanol, and prepare a solution with a concentration of 1mg / mL; absorb 2μL of the solution and place a sample on silica gel GF254 thin-layer plate C, and shake off the ethanol for later use .
[0046] Preparation of linoleic acid solution: Accurately measure 0.6 mL of linoleic acid, dissolve it in 20 mL of n-hexane, and prepare a...
Embodiment 3
[0051] Preparation of the test solution: Accurately weigh 1 mg of water-soluble vitamin E (Trolox), dissolve it in 1 mL of absolute ethanol, and prepare a solution with a concentration of 1 mg / mL.
[0052] Preparation of linoleic acid solution: Accurately measure 0.2, 0.4, 0.6, 0.8 and 1.0mL of linoleic acid, dissolve them in 20mL of n-hexane respectively, and prepare linoleic acid n-hexane solutions of different concentrations (recorded as linoleic acid in sequence acid solutions 1, 2, 3, 4, 5).
[0053] Preparation of developer solution: Add 0.87g of thiobarbituric acid and 2.5g of trichloroacetic acid into 100mL of 50% ethanol aqueous solution, stir to dissolve and mix evenly.
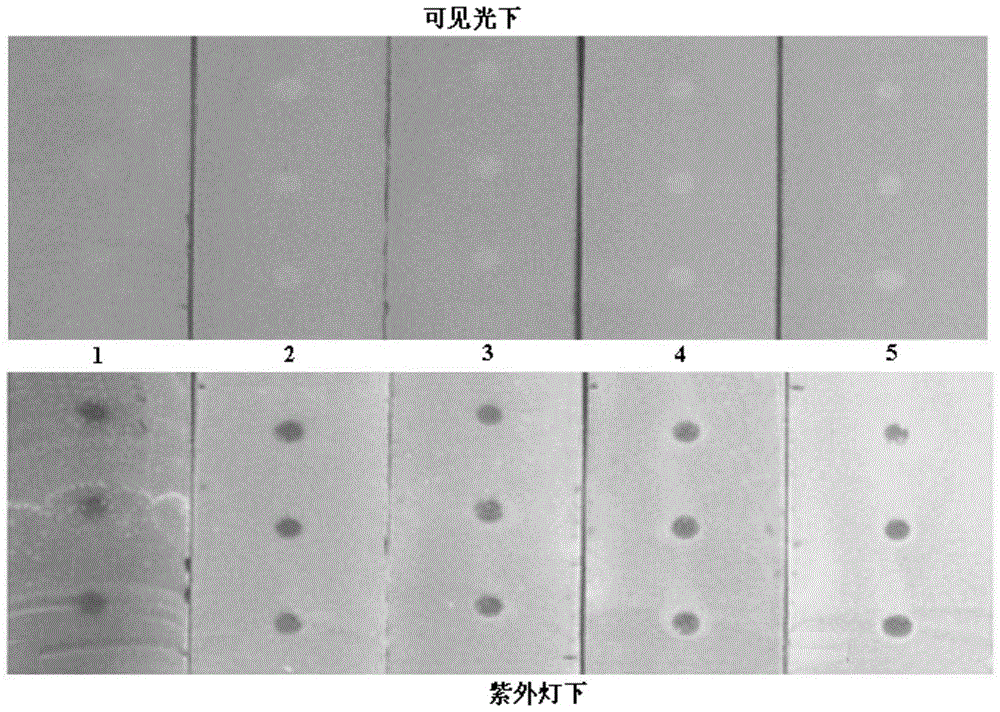
[0054] Take the test solution and place samples in parallel on 5 silica gel GF254 thin-layer plates.
[0055] Immerse the above five thin-layer boards in linoleic acid solutions 1, 2, 3, 4, and 5 respectively (the corresponding thin-layer boards are sequentially recorded as thin-layer boards 1, 2, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com