Novel ultradeep reactive orange or yellow dye and preparation method thereof
A yellow dye, deep reaction technology, applied in reactive dyes, dyeing methods, organic dyes, etc., can solve problems such as unsatisfactory blackness, large performance differences, easy to cause color flowers, etc., to improve absolute color fixation rate and other Various fastnesses, less isomers, and the effect of saving dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
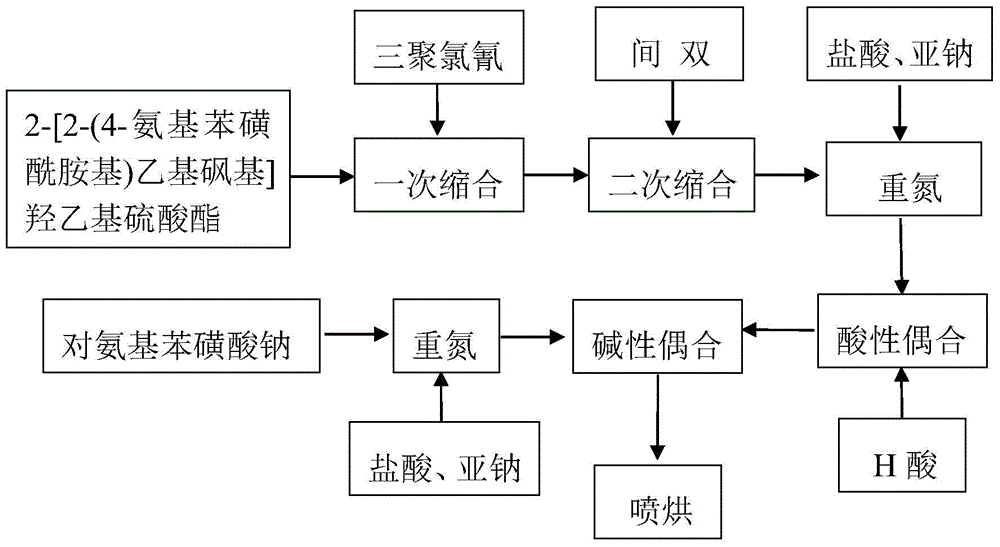
[0043] 1. Condensation reaction for intermediate preparation
[0044] Add 15.8g of acetanilide to 128g of chlorosulfonic acid and react at 40-65°C for 2 hours, add dropwise 26.7g of thionyl chloride and react for 2 hours, dilute and precipitate and filter to obtain 59g (40%) of wet product p-acetamidobenzenesulfonyl chloride .
[0045] Two, thioether synthesis
[0046] Add 13.5g of 2-chloroethylamine hydrochloride to 8.7g of mercaptoethanol solution under stirring, adjust pH=4.8, and react at 55°C for 4 hours to generate 13.2g of β-hydroxyethylsulfide ethylamine. Cool down to 0°C, slowly add 59g (40%) of the tide product p-acetaminobenzenesulfonyl chloride, adjust pH=3.8, control the temperature at 0-2°C for 4 hours, separate and wash to obtain 30.7g p-acetamidobenzenesulfonamide ethyl -2-Hydroxyethyl sulfide.
[0047] 3. Sulfur ether oxidation
[0048] Add 3.15g of sodium tungstate crystals and 6g of water into the three-necked flask, and add 0.45g of 30% hydrochloric aci...
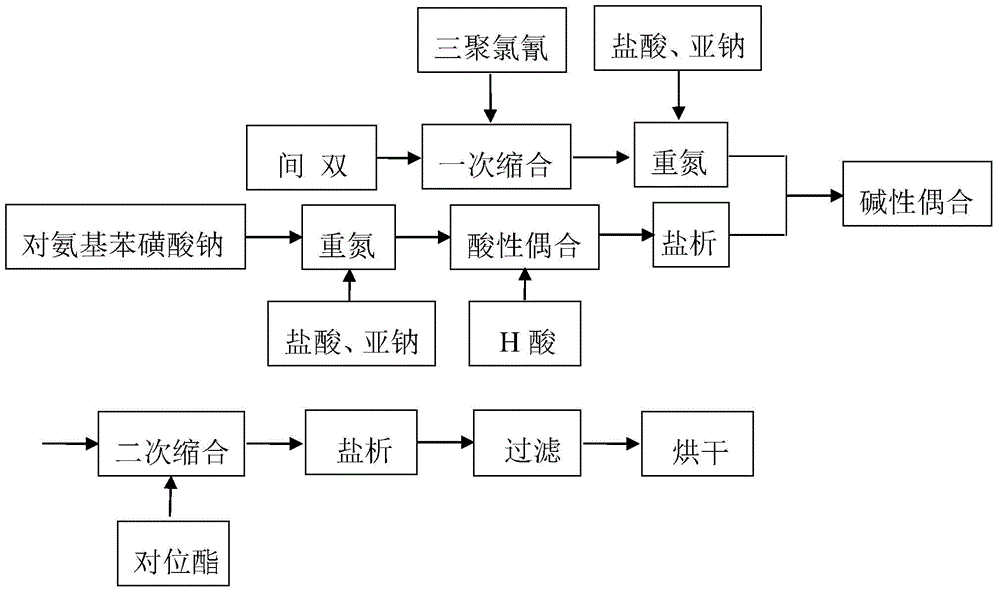
Embodiment 2
[0066] Steps one to nine are exactly the same as in Example 1.
[0067] 10. Diazotization of 2-methoxy-4-(β-hydroxyethylsulfone sulfate) aniline
[0068] Add 10g of water and 80g of ice to a 250ml beaker, add 32.5g of 100% 2-methoxy-4-(β-hydroxyethylsulfone sulfate) aniline under stirring, beat at 0°C for 1 hour, and then add 11g 30% hydrochloric acid, then add 7.11g (97%) industrial sodium nitrite (mixed with 30% sodium nitrite solution) from under the liquid surface, control the temperature at 6°C, KI test paper is slightly blue, and Congo red test paper is deep blue After adding the sodium nitrite solution, keep the temperature at 6°C, and react with a slight blue color of KI test paper for 1 hour. Before coupling, use a small amount of sulfamic acid to eliminate the slight excess of nitrous acid.
[0069] Eleven, triple coupling
[0070]Add 2-methoxy-4-(β-hydroxyethylsulfone sulfate) aniline diazonium salt into the secondary coupling solution that has reached the end poi...
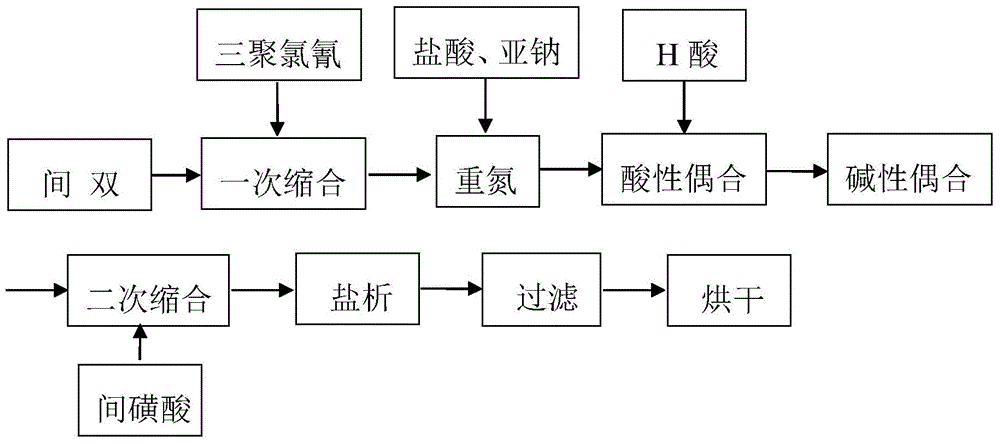
Embodiment 3
[0072] Steps one to nine are exactly the same as in Example 1.
[0073] 10. Diazotization of 2-sulfonic acid-4-(β-hydroxyethylsulfone sulfate) aniline
[0074] Add 10g of water and 140g of ice into a 250ml beaker, add 35g of 2-sulfonic acid-4-(β-hydroxyethylsulfone sulfate) aniline under stirring, beat at 0°C for 1 hour, and then add 6g of 30 % hydrochloric acid, then add 6.98g (97%) industrial product sodium nitrite (being mixed with 30% sodium nitrite solution) from under the liquid surface, control temperature 8 ℃, KI test paper slightly blue, Congo red test paper dark blue, After adding the sodium nitrite solution, keep the temperature at 8°C, and react with a slight blue color of KI test paper for 40 minutes, and use a small amount of sulfamic acid to eliminate the slight excess of nitrous acid before coupling.
[0075] Eleven, triple coupling
[0076] Add 2-sulfonic acid-4-(β-hydroxyethylsulfone sulfate) aniline diazonium salt into the secondary coupling solution that ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com