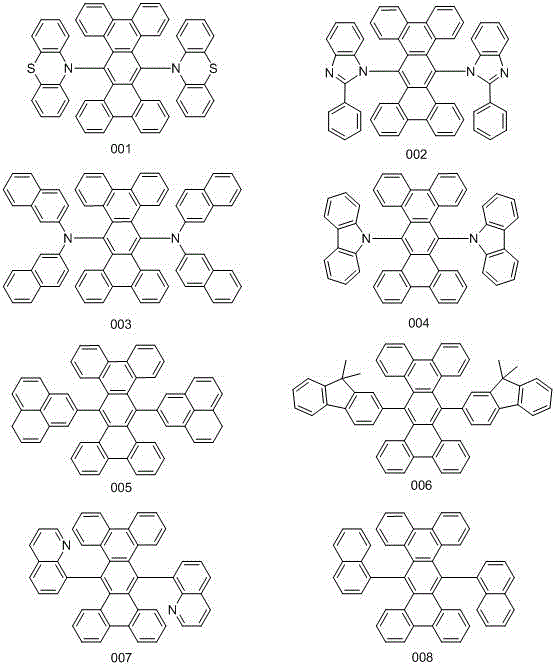
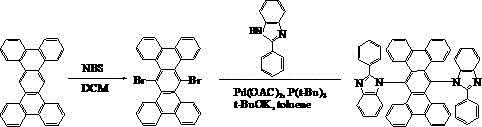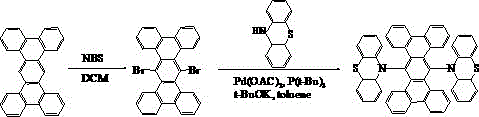Conjugated Derivatives and Their Applications as Electroluminescent Materials
An electroluminescent material and electroluminescent technology, which are applied in the preparation of luminescent materials, organic compounds, hydrocarbons, etc., can solve the problems of not being able to fully satisfy full-color display, not being able to satisfy industrialization, and complex synthesis methods, etc. It is beneficial to vacuum evaporation film formation, improving electron migration ability and high luminous efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0022] Example: Synthesis of Compound 001
[0023] The specific synthetic route is shown in the following formula:
[0024]
[0025] (1) Weigh 18.9 g of tetrabenzotriphenyl, dissolve it in 200 mL of dichloromethane, add 19.58 g of NBS, raise the temperature to 40°C, stir and react for 24 hours, spin dry the organic solvent, and separate the obtained crude product into the column layer. (Petroleum ether / dichloromethane=1:1), as a result, 20.92 g of light yellow solid 6,13-dibromotetrabenzotriphenyl was obtained.
[0026] (2) Dissolve 6,13-dibromotetrabenzotriphenyl, 19.63 g of phenothiazine, 10.94 g of potassium tert-butoxide, 0.09 g of palladium (II) acetate, and 0.08 g of tri-tert-butylphosphorus in 150 ml of toluene, Under nitrogen protection, the reaction was carried out at 100°C for 20 hours. The reaction solution was filtered, the obtained crude product was purified by silica gel chromatography, the obtained solid was recrystallized with toluene, and dried to obtain ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com