Diclofenac copper complexes capable of inhibiting urease activity and preparation method of complexes
A technology of urease activity and diclofenac, applied in copper organic compounds, organic chemical methods, organic chemistry, etc., can solve the problems of short effective inhibition time, low efficiency, poor absorption, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
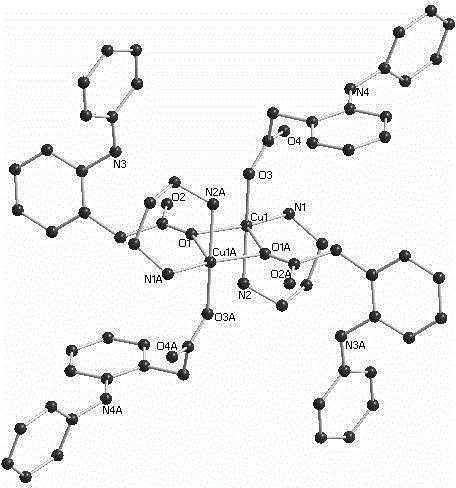
[0020] Example 1: Preparation of a copper complex (complex 1) with diclofenac and 1,3-propanediamine as ligands.
[0021] Preparation of diclofenac sodium solution (0.1mmol / ML methanol): Accurately weigh 0.318g (1mmol) of diclofenac sodium and dissolve in 10mL methanol. Preparation of copper perchlorate solution (0.1mmol / mL methanol): accurately weigh copper perchlorate [Cu(ClO 4 ) 2 .6H 2 O] 0.37g (1mmol) was dissolved in 10mL methanol. Preparation of 1,3-propylenediamine solution (1mmol / mL methanol): Accurately weigh 0.74g (10mmol) of 1,3-propylenediamine and dissolve it in 10mL methanol. Accurately measure 10 mL of diclofenac sodium solution (0.1 mmol / mL methanol) and 5 mL of copper perchlorate solution (0.1 mmol / mL methanol) in a beaker, add methanol to 60 mL, stir at room temperature for 5 minutes, then add 1,3-propane dropwise Diamine solution (1mmol / mL methanol) 0.1mL, sealed with parafilm, pierced with a toothpick on the parafilm, left to stand at room temperature ...
Embodiment 2
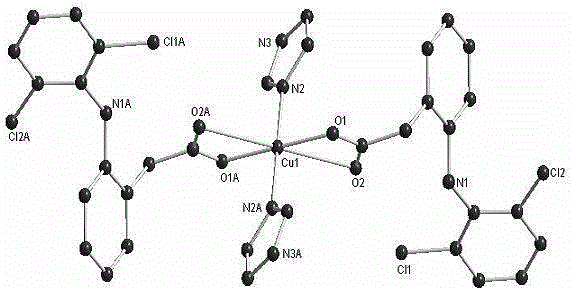
[0024] Example 2: Preparation of a copper complex (complex 2) with diclofenac and imidazole as ligands.
[0025] Preparation of diclofenac sodium solution (0.1 mmol / mL): Accurately weigh 0.318 g (1 mmol) of diclofenac sodium and dissolve in 10 mL of a mixed solvent of methanol and dichloromethane (the volume ratio of methanol to dichloromethane is 1:3). Preparation of copper perchlorate solution (0.1mmol / mL): Accurately weigh copper perchlorate [Cu(ClO 4 ) 2 .6H 2 O] 0.37g (1mmol) was dissolved in 10mL methanol. Preparation of imidazole solution (1mmol / mL): Accurately weigh 0.68g (10mmol) of imidazole and dissolve in 10mL of methanol. Accurately measure 2 mL of diclofenac sodium solution (0.1 mmol / mL methanol: dichloromethane volume ratio of 1:3) and 1 mL of copper perchlorate solution (0.1 mmol / mL methanol) in a beaker, then add dropwise imidazole solution (1 mmol / mL mL methanol) 0.1mL, add a mixed solvent of methanol and dichloromethane (methanol:dichloromethane volume r...
Embodiment 3
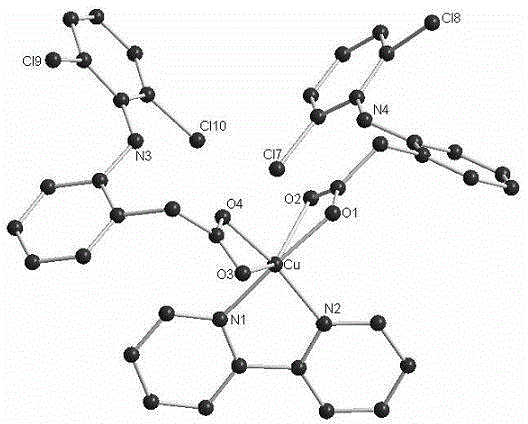
[0028] Example 3: Preparation of a copper complex (complex 3) using diclofenac and 2,2'-bipyridine as ligands.
[0029] The preparation method of diclofenac sodium solution (0.1mmol / mL) and copper perchlorate solution (0.1mmol / mL) is the same as in Example 2. Prepare 1mmol / mL 2,2’-bipyridine solution: Accurately weigh 1.56g (10mmol) of 2,2’-bipyridine and dissolve it in 10mL of methanol. Accurately measure 2mL of diclofenac sodium solution (0.1mmol / mL, the volume ratio of methanol to dichloromethane is 1:3) and 0.1mL of 2,2'-bipyridyl solution (1mmol / mL methanol) in a test tube and mix them evenly. Add 4mL of a mixed solution of methanol and dichloromethane (the volume ratio of methanol to dichloromethane is 1:1) 4mL, then add 1 mL of copper perchlorate solution (0.1mmol / mL methanol) to the test tube, and seal it with a parafilm. The sealing film was pierced with a toothpick, left to stand at room temperature and volatilized slowly. After a few days, green massive crystals pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com