Method for extracting and separating vanadium and chromium from alkaline aqueous solution
An alkaline aqueous solution and an aqueous solution technology are applied in the field of hydrometallurgy, which can solve the problems of inability to extract and separate vanadium and chromium, and achieve the effects of convenient operation, large single-stage separation coefficient and low process cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
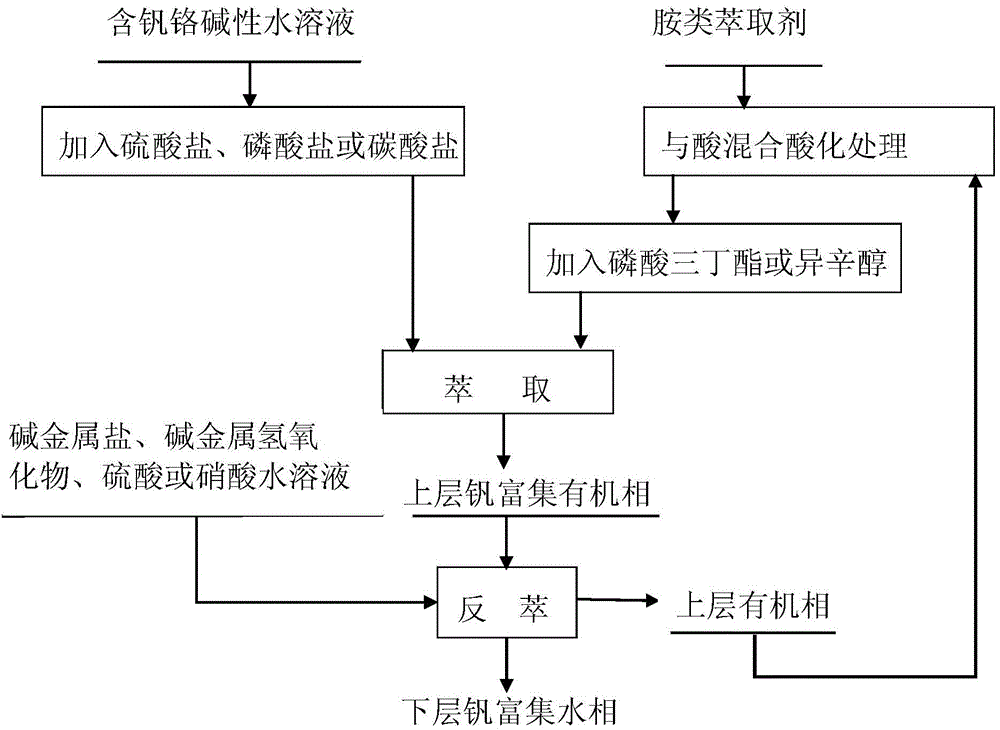
[0047] (1) Add 0.1 g of sodium sulfate to 20 mL of vanadium-chromium-containing alkali metal sodium hydroxide aqueous solution (the mass fraction of sodium hydroxide is 0.01%) to obtain a mixed aqueous solution;
[0048] (2) Fully mix 0.45mol / L hydrochloric acid solution and secondary carbon primary amine according to the volume ratio of 4:1 and let it stand to obtain a system in which the upper and lower layers of liquid coexist. Take the upper organic phase to obtain the acidified secondary Primary carboamines;
[0049] (3) the acidified secondary carboprimary amine and secondary octanol are mixed according to a volume ratio of 1:1 to obtain an organic solution;
[0050] (4) The organic solution and the above-mentioned mixed aqueous solution are mixed and extracted at room temperature according to a volume ratio of 1:1, and then left to stand after being fully mixed to obtain a system in which two layers of liquid coexist, the upper layer is a vanadium-rich organic phase, an...
Embodiment 2
[0055] (1) Add 2.2g of sodium sulfate to 20mL of vanadium-chromium-containing alkali metal sodium carbonate aqueous solution (the massfraction of sodium carbonate is 1%) to obtain a mixed aqueous solution;
[0056] (2) Fully mix 1.5mol / L hydrochloric acid solution and trioctylmethyl ammonium chloride according to the volume ratio of 5:1 and let it stand to obtain a system in which the upper and lower layers of liquid coexist. Take the upper organic phase to obtain the acidified Treated trioctylmethyl ammonium chloride;
[0057] (3) Trioctylmethyl ammonium chloride after acidification treatment is mixed with tributyl phosphate according to a volume ratio of 10:1 to obtain an organic solution;
[0058] (4) The organic solution and the above-mentioned mixed aqueous solution are mixed and extracted at room temperature according to a volume ratio of 1:80, and then left to stand after being fully mixed to obtain a system in which the upper and lower layers of liquid coexist, the upp...
Embodiment 3
[0063] (1) Add 0.15 g of sodium sulfate to 20 mL of vanadium-chromium-containing alkali metal sodium bicarbonate aqueous solution (pH=11) to obtain a mixed aqueous solution;
[0064] (2) After fully mixing 0.5mol / L nitric acid solution and tributyl tertiary amine according to the volume ratio of 6:1, let it stand to obtain a system in which the upper and lower layers of liquid coexist, take the upper organic phase, and obtain the acidified Tributyl tertiary amine;
[0065] (3) tributyl tertiary amine after the acidification treatment is mixed with isooctyl alcohol according to a volume ratio of 2:1 to obtain an organic solution;
[0066] (4) The organic solution and the above-mentioned mixed aqueous solution are mixed and extracted at room temperature according to a volume ratio of 1:50, and then left to stand after being fully mixed to obtain a system in which two layers of liquid coexist, the upper layer is a vanadium-rich organic phase, and the lower layer is chromium enri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com