Method for preparing catalyst for catalytic combustion of methane
A methane catalytic combustion and catalyst technology, applied in physical/chemical process catalysts, chemical instruments and methods, catalyst activation/preparation, etc., can solve the problems of high catalytic temperature, low catalytic efficiency, long preparation period, etc., and achieve good catalytic activity. , the effect of high catalytic activity and short preparation period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
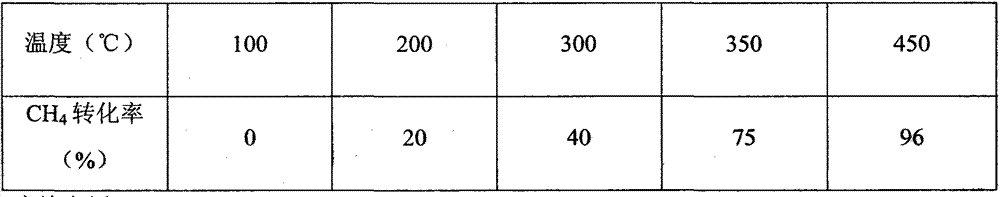
Embodiment 1
[0021] (1) Preparation of cerium oxide / ferric oxide / cobalt oxide (CeO 2 / Fe 2 o 3 / Co 2 o 3 ) carrier:
[0022] Weigh 20-32g, 2.5-3.49g, 0.5-1.44g of cerium nitrate, ferric nitrate and cobalt nitrate respectively, add them into 150ml of distilled water, stir well to obtain mixed salt solution A, and then add 6-10g of Urea (the urea is excessive) is reacted in a closed system, the temperature is 60°C-140°C, and the reaction time is 3-6h. After the reaction, the reactor was naturally cooled to room temperature, and the precipitate was taken out, washed, dried at 105°C for 3 hours, and then calcined at 450°C for 2 hours to obtain a mixed catalyst carrier of various metal oxides.
[0023] (2) Preparation of palladium impregnation solution
[0024] Prepare Pd(NO 3 ) 2 solution, take 0.5mlPd(NO 3 ) 2 solution, add distilled water to 3-6ml, and stir well.
[0025] (3) Loading of active components
[0026] Grind the catalyst carrier prepared in step (1), weigh 6 g of the s...
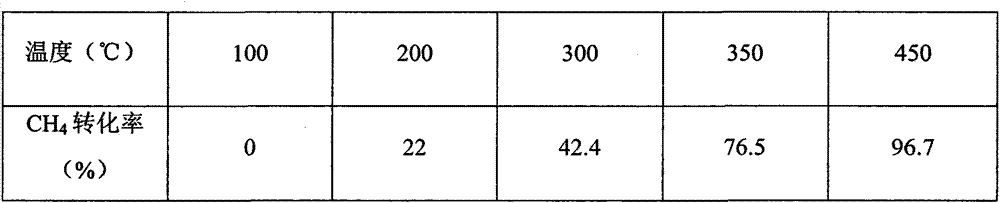
Embodiment 2
[0030] (1) Preparation of cerium oxide / ferric oxide / cobalt oxide (CeO 2 / Fe 2 o 3 / Co 2 o 3 ) carrier:
[0031] Weigh cerium nitrate, ferric nitrate and cobalt nitrate to be 20~32g, 2.5~3.49g, 0.5~1.44g respectively, add into 150ml distilled water, stir well, obtain mixed salt solution A, then add 10g urea ( Where urea is excessive), react in a closed system, the temperature is 60°C-140°C, and the reaction time is 3-6h. After the reaction, the reactor was naturally cooled to room temperature, and the precipitate was taken out, washed, dried at 105°C for 3 hours, and then calcined at 450°C for 2 hours to obtain a mixed catalyst carrier of various metal oxides. where CeO 2 , Fe 2 o 3 and Co 2 o 3 The contents are respectively 90%, 6%, 4%.
[0032] (2) Preparation of palladium impregnation solution
[0033] Prepare Pd(NO 3 ) 2 solution, take 0.5mlPd(NO 3 ) 2 Solution, add distilled water to 6ml, stir well.
[0034] (3) Loading of active components
[0035] Grind...
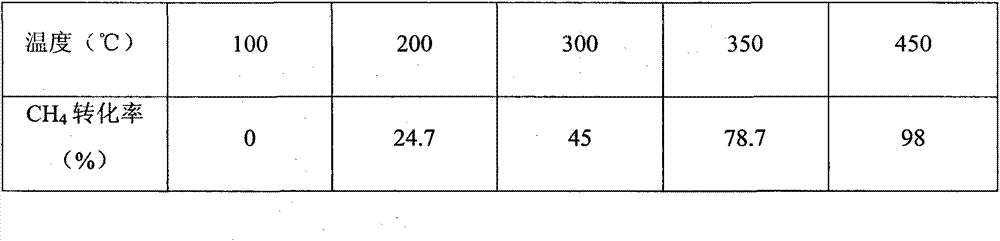
Embodiment 3
[0039] (1) Preparation of cerium oxide / ferric oxide / cobalt oxide (CeO 2 / Fe 2 o 3 / Co 2 o 3 ) carrier:
[0040] Weighing cerium nitrate, ferric nitrate and cobalt nitrate respectively are 32g, 3.49g, 1.44g, join in 150ml distilled water, stir, obtain mixed salt solution A, then add 10g urea (wherein urea is excessive) in solution, in React in a closed system, the temperature is 60°C-140°C, and the reaction time is 3-6h. After the reaction, the reactor was naturally cooled to room temperature, and the precipitate was taken out, washed, dried at 105°C for 3 hours, and then calcined at 450°C for 2 hours to obtain a mixed catalyst carrier of various metal oxides. where CeO 2 , Fe 2 o 3 and Co 2 o 3 The contents are respectively 92%, 5%, 3%.
[0041](2) Preparation of palladium impregnation solution
[0042] Prepare Pd(NO 3 ) 2 solution, take 1ml of Pd(NO 3 ) 2 Solution, add distilled water to 6ml, stir well.
[0043] (3) Loading of active components
[0044] Grin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com