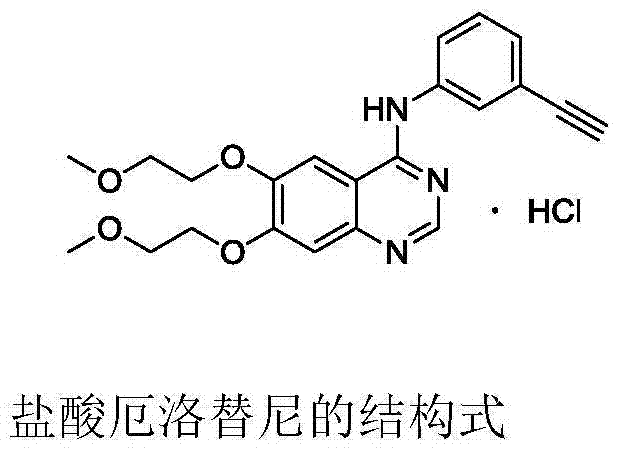
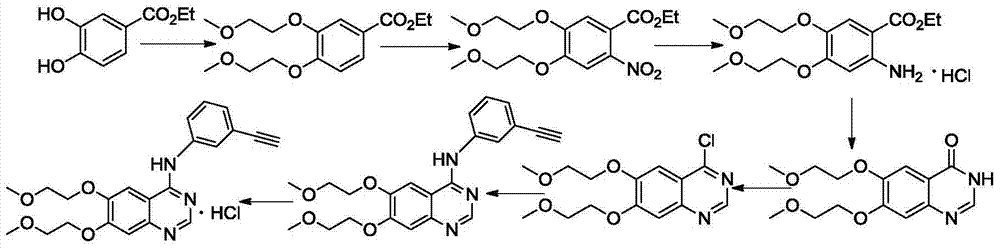
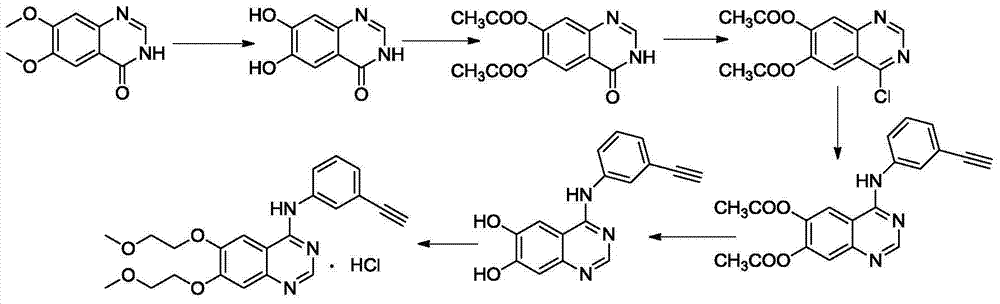
Environment-friendly method for preparing high-yield erlotinib hydrochloride
An erlotinib hydrochloride, high-yield technology, applied in the field of drug synthesis, can solve the problems of low reaction yield, difficult conversion into cyano groups, etc., and achieve the effects of simple post-processing, reduced emissions, and reduced dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Embodiment 1: Preparation of 3,4-dihydroxybenzoic acid ethyl ester
[0066] Put 3,4-dihydroxybenzoic acid (5.0 g, 32.0 mmol) into a reaction flask, add 40 mL of ethanol, heat up to 40° C., and then add 5 mL of concentrated sulfuric acid dropwise. After the dropwise addition, the temperature was raised to 80° C. for reflux reaction for 10 h. After cooling to room temperature, 10M potassium hydroxide solution was added to adjust the pH to neutral. The solvent was distilled off under reduced pressure to precipitate a solid, which was washed with a small amount of water to obtain ethyl 3,4-dihydroxybenzoate.
[0067] Ethyl 3,4-dihydroxybenzoate: white solid; yield 89%; mp: 127~128℃; MS(ESI,calcd / found)[M+H] + : 183.2 / 183.3; 1 H-NMR (DMSO-d 6 )δ:9.52(s,2H),7.35(d,J=2.0Hz,1H),7.31(dd,J 1 =8.0Hz,J 2 =8.0Hz, 1H), 6.80(d, J=8.0Hz, 1H), 4.22(q, J=7.2Hz, 2H), 1.28(t, J=7.2Hz, 3H).
Embodiment 2
[0068] Embodiment 2: the preparation of 3,4-two (2-methoxyethoxy) ethyl benzoate
[0069] Take ethyl 3,4-dihydroxybenzoate (1.7g, 9.3mmol) in a reaction flask, add DMF 8mL to make it completely dissolved, add potassium tert-butoxide (2.0g, 17.8mmol) and potassium iodide (1.0g, 6.0 mmol), after heating up to 100°C, 2-chloroethyl methyl ether (3.52 g, 37.2 mmol) was added dropwise, and the reaction was continued for 12 h after the addition was completed. Cool to room temperature, add ice water (200 mL), and extract three times with ethyl acetate (3×200 mL). The organic phases were combined, washed three times with distilled water, washed three times with saturated brine, dried over anhydrous sodium sulfate, filtered, and the filtrate was evaporated to dryness under reduced pressure to obtain an oil, which was subjected to column chromatography (elution system: petroleum ether / ethyl acetate=6: 1) Obtain ethyl 3,4-bis(2-methoxyethoxy)benzoate.
[0070] Ethyl 3,4-bis(2-methoxyeth...
Embodiment 3
[0071] Embodiment 3: Preparation of 2-nitro-4,5-two (2-methoxyethoxy) ethyl benzoate
[0072] Take ethyl 3,4-bis(2-methoxyethoxy)benzoate (0.4 g, 1.3 mmol) into a reaction flask, add 5 mL of concentrated sulfuric acid, and stir at room temperature to dissolve it. A mixture of fuming nitric acid (0.1 g, 1.3 mmol) and concentrated sulfuric acid (1 mL) was slowly added dropwise in the dark, and stirring was continued for 1 h after the addition was complete. Add ice water (100 mL), extract three times with ethyl acetate (60 ml×3), combine the organic phases, wash three times with saturated sodium bicarbonate and three times with saturated sodium chloride, dry over anhydrous magnesium sulfate overnight, and filter. The filtrate was evaporated to remove the solvent under reduced pressure, and the resulting oil was subjected to column chromatography (elution system: petroleum ether / ethyl acetate = 6:1) to obtain 2-nitro-4,5-bis(2-methoxyethoxy base) ethyl benzoate.
[0073] Ethyl 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com