Diiodostyrene type boron fluoride dipyrrole-hyaluronic acid and its preparation method and application
A technology for substituting boron dipyrrole fluoride and boron dipyrrole fluoride, applied in the field of biomaterials, can solve problems such as large side effects, inability to distinguish normal cells from tumor cells, and poor active targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] (1) Synthesis of 4-(3-hydroxyl-1-propoxy)-1-benzaldehyde
[0055] p-Hydroxybenzaldehyde (0.1mol, 12.2g), 3-bromo-1-propanol (0.11mol, 15.2g) and potassium carbonate (0.3mol, 40g) were added to acetone (200ml). Stir continuously at 60°C until the p-hydroxybenzaldehyde is completely reacted and the reaction is stopped, and the reaction time is 12 hours. The reaction mixture was suction filtered to obtain the filtrate, which was spin-dried and washed with petroleum ether: ethyl acetate = 1:2 as the eluent through silica gel chromatography to obtain the yellow liquid compound 14-(3-hydroxy-1-propoxy) - 1-Benzaldehyde (10.32g), the yield is about 68%. 1 HNMR (400Hz, CD 3 OD)δ9.75(s,1H),7.73-7.70(m,2H),6.93-6.89(m,2H),4.13-4.09(m,2H),3.80-3.76(m,2H),2.01-1.97 (m,2H); ESI-MS: 166.45[M + ].
[0056] (2) Synthesis of boron fluoride dipyrrole
[0057] Benzoyl chloride (1 g, 7.86 mmol) and 2,4-dimethylpyrrole (2.0 mL, 19.65 mmol) were dissolved in dichloromethane (100 mL) un...
Embodiment 2
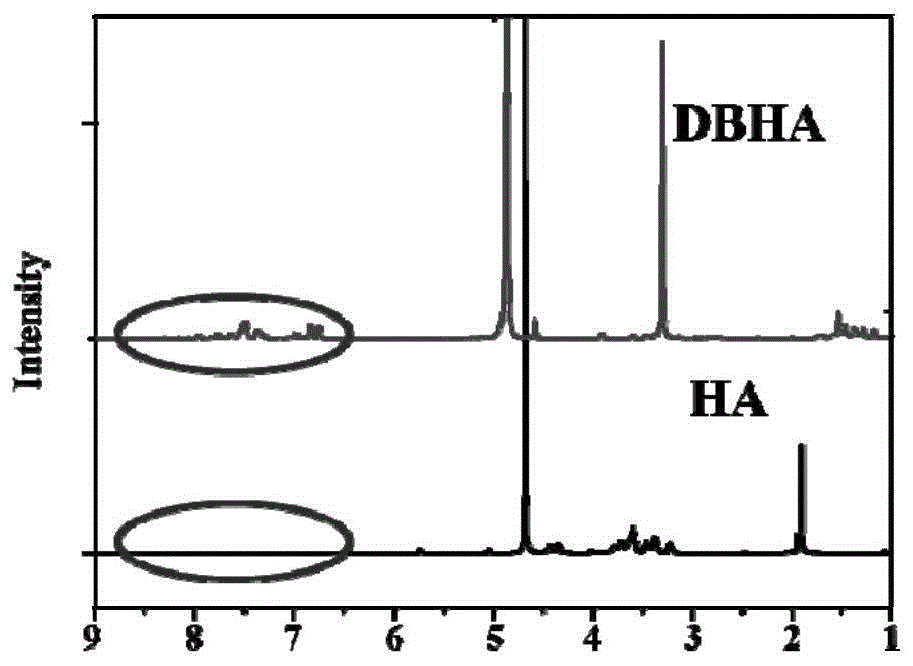
[0067] Diiodostyrene-type boron fluoride dipyrrole-hyaluronic acid nanoparticles and hyaluronic acid were dissolved in D 2 O, and then carry out the proton nuclear magnetic spectrum test to determine the change of the hydrogen chemical shift after the diiodostyrene boron fluoride dipyrrole is attached to the hyaluronic acid. Such as figure 1 As shown, by comparing the NMR spectra of diiodostyrene-type boron fluoride dipyrrole-hyaluronic acid nanoparticles and hyaluronic acid, it can be seen that the chemical shift is between 7 and 8, and the diiodostyrene-type fluoride The boron dipyrrole-hyaluronic acid nanoparticles have a peak, but the pure hyaluronic acid does not. This is due to the chemical shift of the benzene ring after the introduction of the diiodostyrene-type boron dipyrrole on the hyaluronic acid. It can be determined that the two Iodostyrene-type boron fluoride dipyrrole was successfully attached to hyaluronic acid.
Embodiment 3
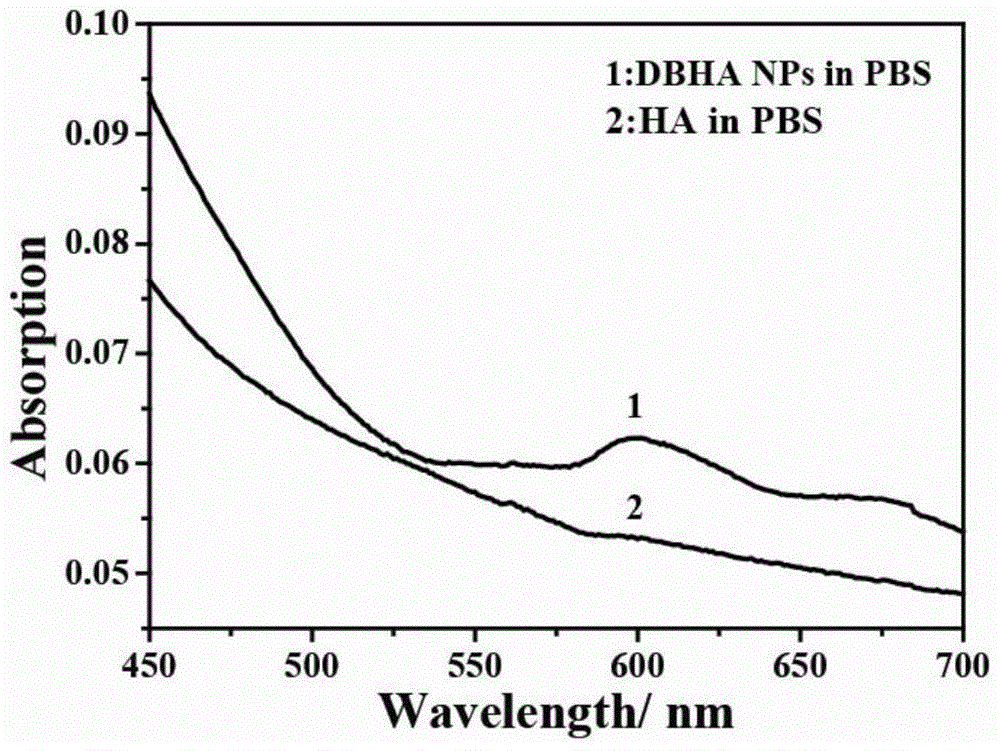
[0069] The ultraviolet-visible absorption spectrum and fluorescence emission spectrum of diiodostyrene boron fluoride dipyrrole-hyaluronic acid nanoparticles were tested by Shimadzu UV-3150 ultraviolet-visible spectrometer and RF-530XPC fluorescence spectrometer. figure 2 For its ultraviolet-visible absorption spectrum, diiodostyrene-type boron fluoride dipyrrole-hyaluronic acid nanoparticles have absorption at 600nm, but pure hyaluronic acid does not, which is diiodostyrene-type boron fluoride UV-Vis absorption of diiodostyrene boron fluoride dipyrrole attached to hyaluronic acid;
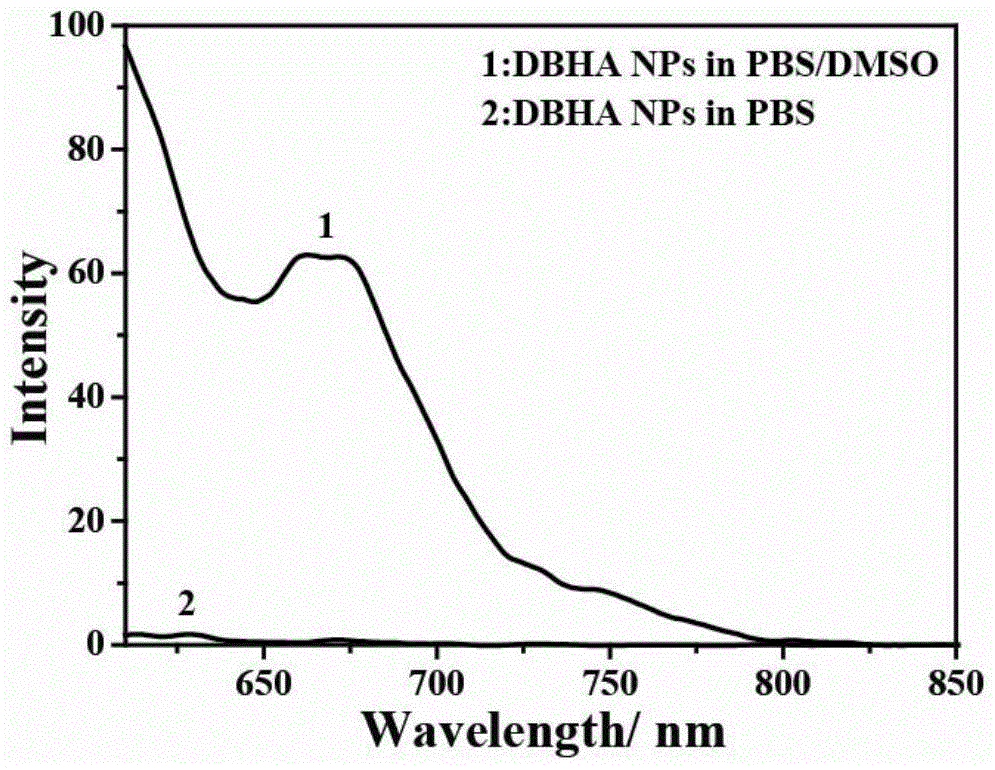
[0070] image 3 It is the fluorescence emission spectrum of diiodostyrene-type boron fluoride dipyrrole-hyaluronic acid nanoparticles in PBS solution, PBS and DMSO mixed solution (volume ratio, 1:1), comparing the two, it can be found that D2 The fluorescence emission of iodostyrene-type boron fluoride dipyrrole-hyaluronic acid nanoparticles in PBS solution is very weak, which is due to the fluo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com