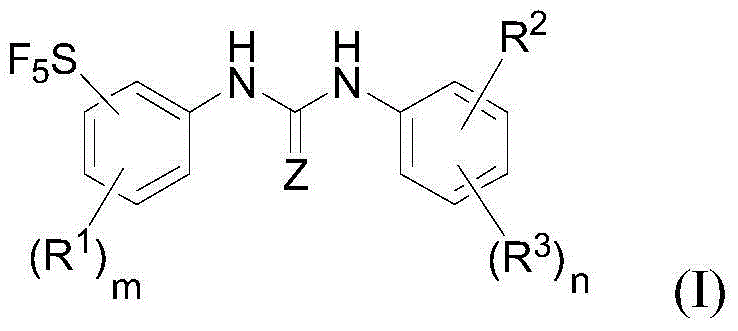
Raf kinase inhibitor pentafluoride sulfur-based aryl urea, and preparation method and applications thereof
An aryl and heteroaryl technology, applied in the field of aryl urea compounds, can solve problems such as drug resistance limitation, and achieve the effect of excellent thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
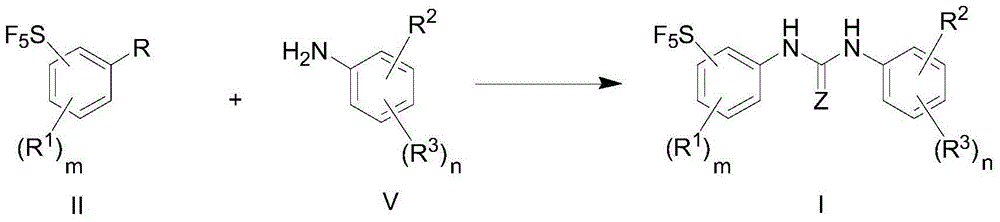
[0086] The preparation method of the compound of formula I of the present invention comprises the step of generating the compound of formula I through the coupling reaction between the compound of formula II and the compound of formula V:
[0087]
[0088] Among the various types, Z, R 1 , R 3 , R 2 , m, n are defined as previously mentioned;
[0089] R is selected from: -NH 2 , -NCS, -NCO, -NHC(=O)R 6 , R 6 Selected from: H, hydroxyl, halogen, nitro, cyano, C1-C6 alkyl, C1-C6 alkoxy, C6-C20 aryl, C3-C8 cycloalkyl, C1-C10 heterocycloalkyl, C5 -C20heteroaryl.
[0090] In another preferred embodiment, R is -NH 2 , the compound of formula X and the compound of formula V undergo a coupling reaction under the action of 1,1'-carbonyl-diimidazole to generate the compound of formula I.
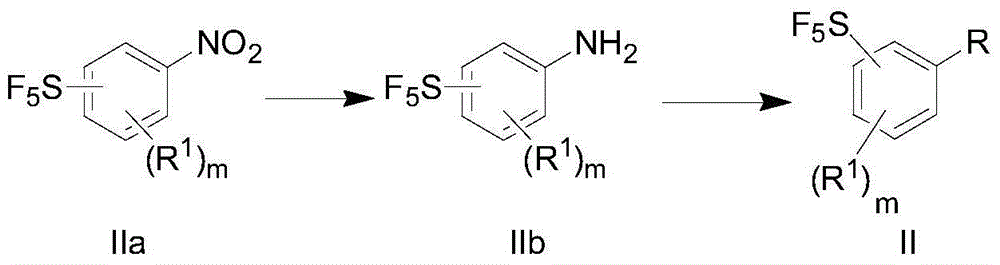
[0091] In another preference, the preparation of the compound of formula II may comprise the following steps:
[0092]
[0093] (a) the compound of formula IIa is reduced to generate th...
Embodiment 1
[0146] Preparation of 3-bromo-5-(pentafluorothio)-aniline:
[0147]
[0148] step one:
[0149] 3-(Pentafluorothio)-nitrobenzene (748 mg, 3 mmol) was dissolved in trifluoroacetic acid TFA (1.5 mL) and concentrated H 2 SO 4 (7.5 mL) of the mixed solvent, vigorously stirred. N-bromosuccinimide NBS (800 mg, 4.5 mml) was added in batches and reacted overnight at room temperature.
[0150] After the reaction, the reaction solution was put into ice water, CH 2 Cl 2 Extract three times, combine the organic phases, and wash three times with saturated brine. Dry over anhydrous sodium sulfate. Filtration, removal of the solvent under reduced pressure, the crude product was purified by flash column chromatography (ethyl acetate / petroleum ether=1 / 5) to obtain a yellow solid 3-bromo-5-(pentafluorosulfuryl)-nitrobenzene (870mg, 89 %Yield).
[0151] 1 H NMR (300MHz, CDCl 3 )δ8.56(t,J=1.9Hz,1H),8.54(t,J=1.8Hz,1H),8.22(t,J=1.9Hz,1H); 19 F NMR (282MHz, CDCl 3 )δ80.81-78.58 (m, 1F...
Embodiment 2
[0156] Preparation of N-methyl-2-pyridinecarboxamide derivatives
[0157]
[0158] 3.12 g of 4-chloro-2-pyridinecarboxylic acid (20 mmol), 1.65 g of methylamine hydrochloride (24 mmol), 3.50 g of 1-(3-dimethylaminopropyl)-3-ethylcarbodiene Amine (23 mmol), 3.0 grams of 1-hydroxybenzotriazole (22 mmol), and 4.0 mL of triethylamine were added to dichloromethane (80 mL). After the reaction mixture was stirred at room temperature for 16 hours, the solvent was removed by rotary evaporation under reduced pressure, and the solid was purified by column chromatography to obtain 4-chloro-N-methyl-2-pyridinecarboxamide (2.96 g, 87% yield of white solid) Rate): MS(ESI+):m / e=171(M+1).
[0159]
[0160] 4.04 g of 4-bromo-2-pyridinecarboxylic acid (20 mmol), 1.65 g of methylamine hydrochloride (24 mmol), 3.50 g of 1-(3-dimethylaminopropyl)-3-ethylcarbodiene Amine (23 mmol), 3.0 grams of 1-hydroxybenzotriazole (22 mmol), and 4.0 mL of triethylamine were added to dichloromethane (80 mL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com