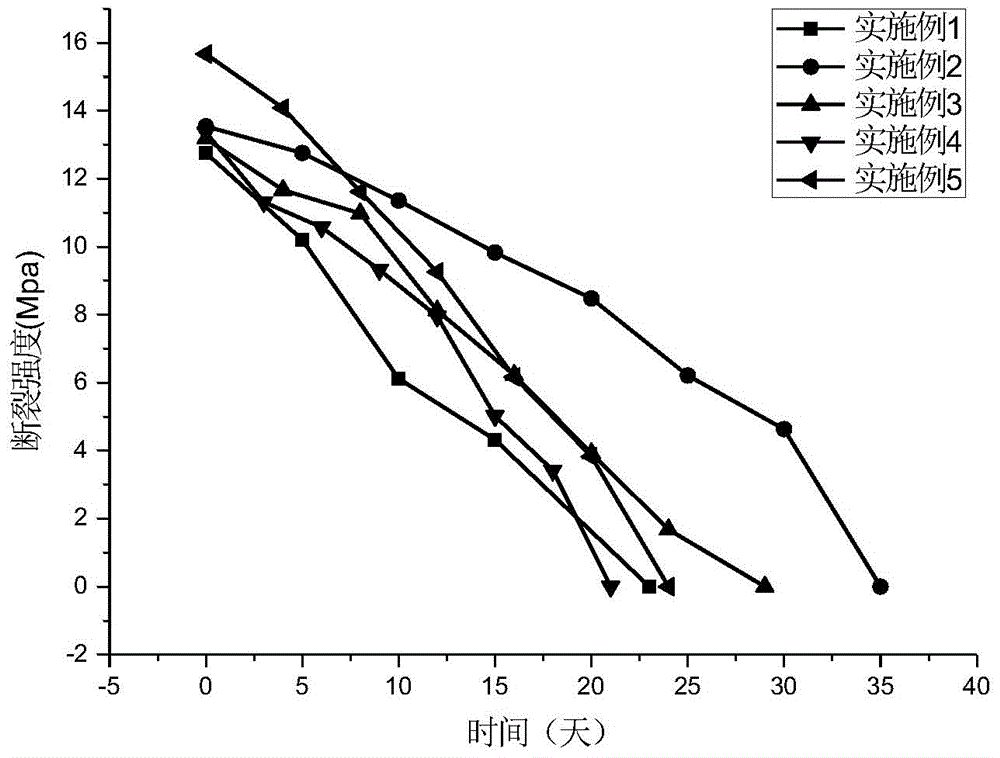
Preparation method of biodegradable polyurethane film material
A polyurethane film and biodegradation technology, which is applied in non-active ingredients medical preparations, pharmaceutical formulations, pharmaceutical sciences, etc., can solve the problems of high raw material cost, many raw materials, and inability to adjust, and achieves simple and easy-to-control process and simple operation. , cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] 1) Synthesis of double-terminated hydroxyl prepolymer
[0092] Put 12.0g (0.02mol) of PEG 600 in a vacuum reaction flask, stir with a magnet, remove water under vacuum (20Pa) for 4 hours at a reaction temperature of 100°C, cool to room temperature, and balance nitrogen into the vacuum reaction flask. Then, 18 g of L-lactide, 18 g of ε-caprolactone and 80 μL of stannous octoate (0.2% of the raw material mass) were added into the vacuum reaction flask. Then vacuumize the vacuum reaction bottle, and then pass dry nitrogen to balance, repeat three times. Finally, the vacuum was evacuated to 20 Pa, the vacuum reaction bottle was sealed, and the oil bath was heated to 140° C., and the double-terminated hydroxyl prepolymer was obtained after 48 hours of reaction.
[0093] 2) Preparation of double-terminated isocyanate-based prepolymer
[0094] Add 20.16g (0.12mol) of HDI to the reaction flask, and add the double-terminated hydroxyl prepolymer synthesized in step 1 dissolved ...
Embodiment 2
[0100] 1) Preparation of double-terminated hydroxyl prepolymer: put 16.0g (0.02mol) PEG 800 in a vacuum reaction bottle, stir with a magnet, remove water at a reaction temperature of 100°C under vacuum (30Pa) for 4 hours, then cool to room temperature, Into the vacuum reaction vial nitrogen balance. Then 18 g of L-lactide, 18 g of ε-caprolactone and 0.14 g of dibutyltin diacetate were added into the vacuum reaction flask. Then vacuumize the vacuum reaction bottle, and then pass dry nitrogen to balance, repeat three times. Finally, the vacuum was evacuated to 30 Pa, the vacuum reaction bottle was sealed, and the oil bath was heated to 150° C., and the double-terminated hydroxyl prepolymer was obtained after 24 hours of reaction.
[0101] 2) Preparation of double-ended isocyanate-based prepolymers:
[0102] Add 23.52g (0.14mol) of HDI to the reaction flask, and add the double-terminated hydroxyl prepolymer synthesized in step 1 dissolved in dioxane (dissolving 1g of prepolymer...
Embodiment 3
[0108] 1) Preparation of double-terminated hydroxyl prepolymer:
[0109] Put 20.0g (0.02mol) of PEG 1000 in a vacuum reaction flask, stir with a magnet, remove water in a vacuum (30Pa) at a reaction temperature of 100°C for 4 hours, cool to room temperature, and pass nitrogen gas into the vacuum reaction flask to balance. Then, 10 g of glycolide, 25 g of ε-valerolactone and 0.14 g of dibutyltin diacetate were added into the vacuum reaction flask. Then vacuumize the vacuum reaction bottle, and then pass dry nitrogen to balance, repeat three times. Finally, the vacuum was evacuated to 20 Pa, the vacuum reaction bottle was sealed, and the oil bath was heated to 120° C., and the double-terminated hydroxyl prepolymer was obtained after 72 hours of reaction.
[0110] 2) Preparation of double-ended isocyanate-based prepolymers:
[0111] Add 16.8g (0.12mol) of BDI into the reaction flask, and add the double-terminated hydroxyl prepolymer synthesized in step 1 dissolved in dioxane (d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| breaking strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com