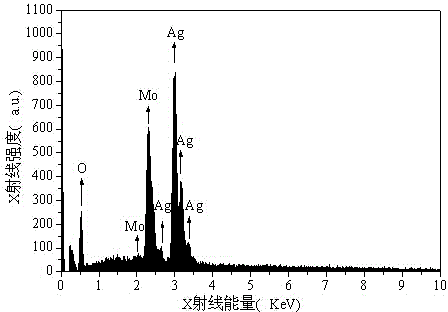
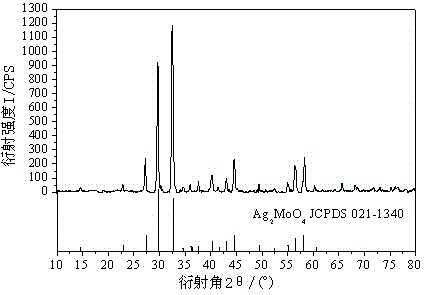
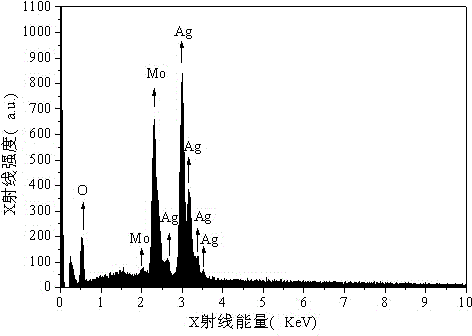
Method for preparing silver molybdate with cationic membrane electrolysis method
A cationic membrane, silver molybdate technology, applied in the electrolysis process, electrolysis components and other directions, can solve the problems of long (up to several hours or even days, the reaction time of additives, etc., to achieve short reaction time, reduced preparation cost, easy effect of operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A kind of method utilizing cationic membrane electrolysis to prepare silver molybdate, specifically comprises the steps:
[0037] (1) Use silver sheet as anode, inert electrode as cathode, 95ml aqueous solution containing depolarizer and sodium molybdate as anolyte, 90ml acid solution as catholyte, double-chamber electrolysis with cationic membrane as diaphragm In the tank, adopt the method of constant voltage electrolysis, control the temperature range from room temperature to 50°C, and constant voltage electrolyze the aqueous solution containing depolarizer and sodium molybdate for 20 minutes;
[0038] The inert electrode is a titanium mesh;
[0039] In the aqueous solution containing depolarizer and sodium molybdate, the concentration of sodium molybdate is 0.1mol / L, the concentration of depolarizer is 0.01mol / L, and the depolarizer is nitric acid;
[0040] Described acid solution is the aqueous hydrochloric acid solution that concentration is 0.1mol / L;
[0041] Th...
Embodiment 2
[0047] A kind of method utilizing cationic membrane electrolysis to prepare silver molybdate, concrete steps are as follows:
[0048] (1) Use silver sheet as anode, inert electrode as cathode, 95ml aqueous solution containing depolarizer and sodium molybdate as anolyte, 90ml acid solution as catholyte, double-chamber electrolysis with cationic membrane as diaphragm In the tank, adopt the method of constant current electrolysis, and control the temperature range from room temperature to 50°C to electrolyze the aqueous solution containing depolarizer and sodium molybdate for 20 minutes;
[0049] The inert electrode is a titanium mesh;
[0050] In the aqueous solution containing depolarizer and sodium molybdate, the concentration of sodium molybdate is 0.1mol / L, the concentration of depolarizer is 0.01mol / L, and the depolarizer is nitric acid;
[0051] Described acid solution is the aqueous hydrochloric acid solution that concentration is 0.1mol / L;
[0052] The cationic membrane ...
Embodiment 3
[0058] A kind of method utilizing cationic membrane electrolysis to prepare silver molybdate, specifically comprises the steps:
[0059] (1) Use silver sheet as anode, inert electrode as cathode, 95ml aqueous solution containing depolarizer and sodium molybdate as anolyte, 90ml alkali solution as catholyte, double-chamber electrolysis with cationic membrane as diaphragm In the tank, adopt the method of constant current electrolysis, and control the temperature range from room temperature to 50°C to electrolyze the aqueous solution containing depolarizer and sodium molybdate for 20 minutes;
[0060] The inert electrode is a titanium mesh;
[0061] In the aqueous solution containing depolarizer and sodium molybdate, the concentration of sodium molybdate is 0.1mol / L, the concentration of depolarizer is 0.01mol / L, and the depolarizer is sodium nitrate;
[0062] Described alkali solution is the sodium hydroxide aqueous solution that concentration is 0.1mol / L;
[0063] The cationi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com